Scroll to:
Pharmacogenetic markers in the treatment of patients with multidrug-resistant tuberculosis
https://doi.org/10.37489/2588-0527-2025-2-23-29
EDN: UISFIM
Abstract
Background. Treatment of patients with tuberculosis (TB) with multidrug-resistant (MDR) causative agent is often complicated by adverse reactions (AR) with forced drug discontinuation, its effectiveness is far from the target indicators and depends on a number of factors, including the patient's genetic characteristics. Pharmacogenetic markers of MDR-TB have not been studied; it is expected that their identification will improve the results of treatment based on a personalized approach.
Objective. to determine the pharmacogenetic markers associated with the efficacy and safety of treatment of patients with MDR TB.
Methods. A prospective cohort study included 40 patients with MDR-TB without HIV infection who received therapy with bedaquiline, linezolid, and a fluoroquinolone in 2023–2024. All patients had 3–5 ml of venous blood collected once, regardless of the duration of therapy. Real-time PCR was used to determine the presence of single-nucleotide polymorphisms in the genes for cytochromes (CYP3A4, CYP3A5), P-glycoprotein (ABCB1), the membranebound ATP-binding cassette transporter (ABCG2), and the organic anion transporter (SLCO1B1), which were selected based on literature analysis and the PharmGKB database. The relationship between these indicators and the effectiveness and safety of treatment was assessed using univariate analysis, with the calculation of the odds ratio (OR) and its 95 % confidence interval (CI).
Results. Target polymorphisms were identified: SLCO1B1 (rs4149056 — in 25.8 %), ABCB1 (rs1045642 — in 75.0 %, rs2032582 — 72.2 %, rs1128503 — 77.8 %), ABCG2 (rs2231142 — in 24.3 %), CYP3A4 (rs2740574 — in 8.1 %), CYP3A5 (rs776746 — in 10.8 %). The treatment efficacy based on the criterion of cessation of bacteriosis was 89.3 % (95 % CI 72.0–97.1 %); the incidence of adverse events was 70 % (95 % CI 54.5–82.0 %), with neurotoxic reactions prevailing (in 11 of 40 patients, 27.5 %). The AA genotypes of the CYP3A5 rs776746 gene and the AA genotypes of the ABCG2 rs2231142 gene were associated with a minimum frequency of cessation of bacterial shedding: respectively, in 33 % and 0% of individuals with each variant, compared to 100% in the rest, p < 0.01; OR 0.021 (95 % CI 0.001–0.77) and 0.083 (95% CI 0.01–0.98). The risk of neurotoxic reactions was higher in the presence of the "wild" variant (genotype GG) of the ABCB1 rs2032582 gene (55.6 % vs. 16.0 % in patients with allelic polymorphisms, p = 0.034; OR 6.3; 95 % CI 1.2–33.3); gastrointestinal reactions — in the presence of the TT genotype of the ABCB1 rs1128503 gene (50.0 % vs. 10.0 %, p = 0.045; OR=9.0; 95 % CI 1.22–66.2 %).
Conclusion. Polymorphisms of CYP3A5 (rs776746, AA genotype) and ABCG2 (rs2231142, AA genotype) genes were revealed, associated with unfavorable results of treatment of patients with MDR-TB. Genetic predictors of neurotoxic and gastrointestinal reactions during treatment of patients with MDR of the pathogen were determined.
Keywords
For citations:
Ivanova D.A., Yurovskaya E.I., Galkina K.Yu. Pharmacogenetic markers in the treatment of patients with multidrug-resistant tuberculosis. Pharmacogenetics and Pharmacogenomics. 2025;(2):23-29. (In Russ.) https://doi.org/10.37489/2588-0527-2025-2-23-29. EDN: UISFIM
Introduction
Despite a significant improvement in the global and Russian Federation tuberculosis (TB) epidemiological situation, the treatment of patients with multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis remains a relevant challenge. The treatment success rate remains far from the target values: according to WHO data, for Russian patients with pre-XDR and XDR tuberculosis in 2024, it was 54% (against a target level of 80%) [1]. This gap necessitates large-scale efforts to address the problem, and one promising direction is the development of personalized patient management strategies that account for individual variations in treatment response [2, 3].
It is postulated that the pharmacological response to treatment is equally dependent on phenotypic factors (sex, age, body weight, race, nature of the tuberculous process, nutritional status, comorbidities, immune dysfunction, drug-drug interactions) and genetic factors that determine the activity of enzymes and transporters involved in the biotransformation of anti-tuberculosis drugs and immune response mediators. Identifying these factors (polymorphisms in the corresponding genes), along with considering phenotypic characteristics, will allow for the selection of optimal anti-tuberculosis drug (ATD) regimens and dosages for the patient, thereby improving treatment outcomes while minimizing the risk of toxic effects [4–7].
The composition of modern TB treatment regimens differs drastically depending on the presence of MDR in the causative pathogen. While a number of potential pharmacogenetic markers are known for drug-susceptible tuberculosis [8–11], and clinical practice utilizes the assessment of acetylation type (N-acetyltransferase 2 genotype, a key participant in isoniazid metabolism), biomarkers for patients with MDR/pre-XDR/XDR tuberculosis remain understudied, and a personalized strategy has not been developed.
When searching for genetic polymorphisms as candidate pharmacogenetic biomarkers, the following conditions must be considered: 1) the encoded protein's involvement in the pharmacokinetics of the drug(s); 2) an association between the presence of the polymorphism and clinical effect or the risk of adverse reactions (AR); 3) population frequency (not less than 1%); 4) potential utility for dose adjustment [5].
The "core" of modern MDR/XDR-TB chemotherapy regimens includes so-called Group A drugs—bedaquiline, linezolid, levofloxacin, or moxifloxacin; dose adjustment is primarily justified for linezolid and fluoroquinolones. The metabolism and excretion of these drugs involve a range of enzymes and transporters. Key among them are cytochrome P450 isoforms CYP3A4 and CYP3A5, which participate in the metabolism of linezolid and bedaquiline, and three major transporter proteins [12–14]: P-glycoprotein, ATP-binding cassette transporter G2, and the organic anion transporter polypeptide OATP1B1 (gene SLCO1B1). The encoding genes for each of these proteins are known, and mutations in them may be associated with altered pharmacological response. The potential for utilizing this information in phthisiatric practice for predicting and managing treatment response remains unknown.
Objective: To determine pharmacogenetic markers associated with the efficacy and safety of treatment in patients with MDR-TB.
Methods
A prospective cohort study included 40 patients with MDR-, pre-XDR-, and XDR-TB without HIV infection, registered for a treatment course containing linezolid, a fluoroquinolone, and bedaquiline in 2023–2024 at hospitals of the Moscow City Scientific and Practical Center for Tuberculosis Control. The cohort consisted of 24 men (60.0%) and 16 women (40.0%) aged 19–66 years (median 42 years, interquartile range [IQR] 32.2–48.0 years). TB was newly diagnosed in 24 patients (60%). Infiltrative TB was the predominant clinical form (57.5%); the proportion of patients with disseminated TB was 17.5%, with pulmonary tuberculoma 15%, and with fibrocavernous TB and caseous pneumonia 5% each (2 patients each). Cavitary lesions were identified in 29 patients (72.5%), and bacterial shedding at the start of chemotherapy was present in 28 patients (70%). MDR was confirmed by microbiological and molecular genetic methods in 23 patients (73%), pre-XDR in 7 (17%), and XDR in 4 patients (10%). Comorbidities were present in 35 out of 40 patients (87.5%), with pathology of the central nervous system (encephalopathy of various origins, 17 patients, 42.5%), gastrointestinal tract (32.5%), musculoskeletal system (30%), and cardiovascular system (25%) being the most prevalent.
The chemotherapy regimen was formed according to the current version of clinical guidelines ("Tuberculosis in Adults" [15]), considering the individual drug susceptibility profile of the pathogen, anamnestic data on therapy tolerance, and the spectrum and severity of comorbidities. All patients received bedaquiline, linezolid, and a fluoroquinolone (moxifloxacin, levofloxacin, or sparfloxacin) as part of their treatment regimen, along with other drugs recommended for the regimen (cycloserine or terizidone, delamanid, prothionamide, PAS, amikacin or capreomycin, carbapenems). The spectrum of prescribed ATDs is presented in Fig. 1.

Fig. 1. Frequency of administration of various anti-tuberculosis drugs in 40 patients with respiratory tuberculosis (the percentage of patients who received each drug is indicated).
Based on literature analysis and data from the PharmGKB online knowledge base (https://www.pharmgkb.org/), single nucleotide polymorphisms (SNPs) in the genes CYP3A4, CYP3A5, ABCB1, SLCO1B1, and ABCG2, associated with the pharmacokinetics of key ATDs for MDR-TB treatment (see Table 1), and amenable to testing by real-time polymerase chain reaction (RT-PCR), were identified.
A single 3–5 ml venous blood sample was collected from all patients, irrespective of therapy duration, for pharmacogenetic testing. Using RT-PCR kits produced by Sintol LLC (Russia), the presence of target SNPs in transporter protein genes was determined: SLCO1B1 (rs4149056 or T521C), ABCB1 (rs1045642 or C3435T, rs2032582 or G2677T, rs1128503 or C1236T), ABCG2 (rs2231142, C421A), as well as cytochrome P450 family enzymes: CYP3A4 (rs2740574, A/G), CYP3A5 (rs776746, G/A). The observation period for each patient was at least 6 months (for 38 out of 40 patients, it corresponded to the duration of the intensive phase).
Treatment efficacy was assessed based on the time to cessation of bacterial shedding and the presence of positive clinical and radiographic dynamics (closure of cavities). Safety was assessed based on the frequency and spectrum of adverse reactions, including the presence of grade 3-4 reactions according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (2017) [16]. The causal relationship between a reaction and the intake of a specific drug in the regimen was assessed using the Naranjo scale and expert evaluation. The association between efficacy/safety indicators and the presence/variant of the studied SNPs was determined based on univariate analysis using the χ² test, Fisher's exact test, calculation of odds ratios (OR), and their 95% confidence intervals (95% CI). Statistical analysis was performed using IBM SPSS Statistics, version 25.0.
Results
The intensive phase of treatment was successfully completed with confirmed efficacy in 37 out of 40 patients (92.5%) within 6–9 months; one of the three remaining patients died from progression of the tuberculous process, and two continue treatment with regimen adjustment and prolongation of the intensive phase. Cessation of bacterial shedding was observed in 25 out of 28 patients who were initially culture-positive (89.3%; 95% CI 72.0–97.1%) within 4 to 36 weeks from the start of chemotherapy (median 4 weeks, IQR 4–8 weeks); cavities closed in 23 out of 29 patients (79.3%; 95% CI 61.3–90.5%).
Adverse reactions were registered in 36 patients (90.0%; 95% CI 76.9–96.0%), and in 62.5% of patients (95% CI 47.0–75.8%), grade 3-4 ARs developed, requiring the discontinuation of at least one ATD and regimen correction. A total of 50 AR cases were recorded, ranging from one to six per patient. The spectrum of ARs is presented in Fig. 2.

Fig. 2. Spectrum of adverse reactions (indicated as the percentage of patients with developed reactions).
Neurotoxic reactions (13 patients, 32.5%, predominantly peripheral neuropathy) and gastrointestinal reactions (7 patients, 17.5%) were most common, followed by arthralgias (12 patients, 30%). Hematological and allergic reactions were noted equally (4 cases each, 10%), as were nephro- and hepatotoxic reactions (2 patients each, 5%). Clinically significant QTc interval prolongation (over 500 msec) was observed in 4 patients (10%).
The results of pharmacogenetic testing (frequency of detection of different variants of the studied polymorphisms) are presented in Table 1.
Table 1. Frequency of detection of genetic polymorphisms in the study group (40 patients with tuberculosis).
| Proportion of patients with different genotype variants | OATP1B1 rs4149056 (T521C) | ABCB1 rs1045642 (C3435T) | ABCB1 rs2032582 (G2677T) | ABCB1 rs1128503 (C1236T) | ABCG2 rs2231142 (C421A) | CYP3A4 rs2740574 (A/G) | CYP3A5 rs776746 (G/A) |
|---|---|---|---|---|---|---|---|
| Presence of at least one mutant allele | 25.8% | 75.0% | 72.2% | 77.8% | 24.3% | 8.1% | 10.8% |
| Heterozygote (1 polymorphic allele) | 6.5% | 58.3% | 52.8% | 61.1% | 13.5% | 5.4% | 0.0% |
| Homozygote (both alleles polymorphic) | 19.4% | 16.7% | 19.4% | 16.7% | 10.8% | 2.7% | 10.8% |
| Homozygous wild-type | 74.2% | 25.0% | 27.8% | 22.2% | 78.4% | 91.9% | 89.2% |
The frequency of the studied allelic polymorphisms varied from 8.1% (for the CYP3A4 gene) to 77.8% (for the rs1128503 polymorphism in the P-glycoprotein gene). Genotypes with mutations in both gene alleles (homozygous) were rare (2.7–19.4%); it was presumed that in this case, the phenotype corresponds to the most significant impairment of the encoded protein's function.
An association between treatment efficacy indicators and two pharmacogenetic markers was identified: the presence of homozygous polymorphisms in the cytochrome CYP3A5 (rs776746) and ATP-binding cassette transporter G2 (ABCG2, rs2231142) genes.
Specifically, cessation of bacterial shedding was registered in only one out of three culture-positive patients with the AA genotype of CYP3A5 rs776746 (33%), compared to 100% sputum conversion in 25 patients with the "non-mutant" genotype (GG), p < 0.01, OR = 0.021 (95% CI 0.001–0.77).
None of the patients with a homozygous mutation in the ABCG2 gene (rs2231142, genotype AA) achieved cessation of bacterial shedding within the standard duration of the intensive chemotherapy phase (one patient achieved it after 9 months of treatment), whereas patients with AG and GG genotypes achieved successful treatment outcome by the microbiological criterion in 100% of cases, p < 0.01, OR = 0.083 (95% CI 0.01–0.98). The AA genotype of ABCG2 rs2231142 was also associated with a low frequency of cavity closure: 25% vs. 75% in patients with AG and GG genotypes, p < 0.01, OR = 0.083, 95% CI 0.01–0.98 (Fig. 3).
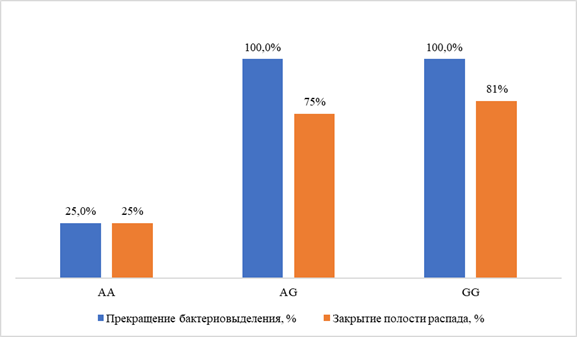
Fig. 3. Treatment efficacy indicators in patients with different ABCG2 rs2231142 genotype variants.
None of the studied polymorphisms demonstrated a statistically significant association with the overall frequency of ARs. When analyzing potential markers for specific types of reactions, a higher frequency of neurotoxic reactions was determined in patients with the GG ("wild-type") genotype of ABCB1 rs2032582 (55.6% vs. 16.0% in patients with AG and AA genotypes), p = 0.034, OR = 6.3 (95% CI 1.2–33.3). The presence of the TT genotype (homozygous mutation) in the ABCB1 rs1128503 gene was a risk factor for gastrointestinal reactions (50.0% vs. 10.0% in patients with CT and CC genotypes), p = 0.045, OR = 9.0 (95% CI 1.22–66.2).
Furthermore, a trend towards an increased risk of hematological reactions (anemia, leukopenia, thrombocytopenia) was observed in the presence of the TT genotype of the ABCB1 rs1045642 gene (33.3% in patients with the homozygous polymorphism vs. 3.3% in patients with CC and CT genotypes); the difference was statistically non-significant—p = 0.06 by χ² test, OR = 14.5 (95% CI 1.06–198.8). Conversely, the presence of the rs4149056 polymorphism (CC or TC genotypes) of the SLCO1B1 gene played a protective role: the frequency of arthralgias was 45.5% in patients with the TT genotype (absence of polymorphism, "wild-type" variant), while no cases of arthralgia were noted in patients with at least one "mutant" allele (p = 0.03 by Fisher's exact test, OR = 1.79, 95% CI 1.23–2.56). Thus, polymorphisms in transporter protein genes, particularly P-glycoprotein, were of greatest importance in the genesis of ARs.
Discussion
This study is exploratory regarding a set of pharmacogenetic markers applicable to modern MDR-TB treatment regimens. Earlier isolated works were devoted to studying the association of individual polymorphisms either with the pharmacokinetics of a specific drug [13, 14] or with clinical indicators of efficacy and safety [17, 18]. Our study did not confirm the association between treatment efficacy, AR frequency, and the CYP3A4 rs2740574 polymorphism identified in the work of Zakharov A.V. et al. [17]; however, data were obtained on the potential utility of a number of other biomarkers associated with the pharmacokinetics of key drugs used in modern MDR-TB regimens. In particular, the role of the CYP3A5 polymorphism, identified in the work of Yunusbaeva M.M. et al. [18], was confirmed. The main result of the study is the identification of a genetic predictor of ineffective treatment—the polymorphism in the ABCG2 gene of the ATP-binding cassette transporter G. Previously, this SNP was associated only with the risk of hepatotoxicity [19]. Given the obtained data, the detection of the homozygous genotype (AA) may guide the physician towards longer treatment regimens.
Furthermore, allelic polymorphisms associated with the risk of significant adverse reactions (for the P-glycoprotein and organic anion transporter 1B1 genes) were identified. Thus, a potential composition for a pharmacogenetic panel applicable for predicting treatment response and developing an optimal therapeutic strategy for the most complex category of patients has been determined.
This study has several limitations related to its "pilot," exploratory nature: primarily, the relatively small sample size, and secondly, the absence of analysis of phenotypic factors with potential influence on treatment outcome. A continuation of the work is planned using a larger sample size and studying the prognostic significance of polymorphisms in other enzymes and transporters associated with the efficacy and safety of treatment in patients with MDR/pre-XDR/XDR tuberculosis.
Conclusion
Polymorphisms in the CYP3A5 (rs776746, genotype AA) and ABCG2 (rs2231142, genotype AA) genes, associated with unfavorable MDR-TB treatment outcomes, were identified. Genetic predictors of neurotoxic and gastrointestinal reactions during the treatment of patients with MDR-TB were determined.
The obtained results are applicable for identifying patients requiring individualized treatment regimens to select the most effective therapeutic tactics, as well as for the early prevention of corresponding types of ARs.
References
1. WHO. Global Tuberculosis Report 2024. – Geneva: World Health Organization, 2024. – P. 1-68. URL: https://worldhealthorg.shinyapps.io/tb_profiles/
2. Guglielmetti L, Panda S, Abubakirov A, et al. Equitable, personalised medicine for tuberculosis: treating patients, not diseases. Lancet Respir Med. 2025 May;13(5):382-385. doi: 10.1016/S2213-2600(25)00080-3.
3. Thu VTA, Dat LD, Jayanti RP, et al. Advancing personalized medicine for tuberculosis through the application of immune profiling. Front Cell Infect Microbiol. 2023 Feb 10;13:1108155. doi: 10.3389/fcimb.2023.1108155.
4. Clinical pharmacogenetics: textbook. a manual for students of medical universities / DA Sychev [et al.]; ed by VG Kukes, NP Bochkov. Moscow: GEOTARMedia, 2007. (In Russ.)]. ISBN 978-5-9704-0458-4.
5. Mozhokina GN, Kazakov AV, Elistratova NA, Popov SA. Biotransformation enzymes for xenobiotics and personalization of treatment regimens for tuberculosis patients. Tuberculosis and Lung Diseases. 2016;94(4):6-12. (In Russ.). doi: 10.21292/2075-1230-2016-94-4-6-12.
6. Verma R, da Silva KE, Rockwood N, Wasmann RE, Yende N, Song T, Kim E, Denti P, Wilkinson RJ, Andrews JR. A Nanopore sequencing-based pharmacogenomic panel to personalize tuberculosis drug dosing. medRxiv. Am J Respir Crit Care Med. 2024 Jun 15;209(12):1486-1496. doi: 10.1164/rccm.202309-1583OC.
7. Kantemirova BI, Galimzyanov KM, Stepanova NA, et al. Prospects of Pharmacogenetic Testing for Design of Algorithms for Personalized Treatment of Tuberculosis of Respiratory Organs in the Astrakhan Region. Antibiotiki i Khimioterapiya = Antibiotics and Chemotherapy. 2015; 60(9-10):29-32. (In Russ.).
8. Azuma J, Ohno M, Kubota R, et al; Pharmacogenetics-based tuberculosis therapy research group. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol. 2013 May;69(5):1091-101. doi: 10.1007/s00228-012-1429-9.
9. Krasnova NM, Evdokimova NE, Egorova AA, et al. Influence of the Acetylation Type on the Incidence of Isoniazid-Induced Hepatotoxicity in Patients with Newly Diagnosed Pulmonary Tuberculosis. Antibiotiki i Khimioterapiya = Antibiotics and Chemotherapy. 2020;65(7-8):31-36. (In Russ.). doi: 10.37489/02352990-2020-65-7-8-31-36.
10. Yang S, Hwang SJ, Park JY, et al. Association of genetic polymorphisms of CYP2E1, NAT2, GST and SLCO1B1 with the risk of anti-tuberculosis drug-induced liver injury: a systematic review and meta-analysis. BMJ Open. 2019;9(8):e027940. doi:10.1136/bmjopen-2018-027940.
11. Ivanova DA, Galkina XYu, Borisov SE, et al. Risk of the hepatotoxicity evaluation by the pharmacogenetic methods in new tuberculosis patients. Tuberculosis and socially significant diseases. 2018;(3):43-48. (In Russ.).
12. Problems of Drug Resistance in Mycobacteria / ed by EM Bogorodskaya, DA Kudlay, VI Litvinov. Moscow: MNPCBT, 2021. (In Russ.). ISBN 978-5-89180-134-9.
13. Haas DW, Abdelwahab MT, van Beek SW, et al. Pharmacogenetics of Between-Individual Variability in Plasma Clearance of Bedaquiline and Clofazimine in South Africa. J Infect Dis. 2022 Aug 12;226(1):147-156. doi: 10.1093/infdis/jiac024.
14. Annisa N, Afifah NN, Santoso P, et al. Pharmacogenetics and Pharmacokinetics of Moxifloxacin in MDR-TB Patients in Indonesia: Analysis for ABCB1 and SLCO1B1. Antibiotics (Basel). 2025 Feb 16;14(2):204. doi: 10.3390/antibiotics14020204.
15. Clinical guidelines. Tuberculosis in adults. 2024. Ministry of Health of the Russian Federation: official website. (In Russ.). Доступно по: https://cr.minzdrav.gov.ru/recomend/16_3. Ссылка активна на 15.06.2025
16. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Доступно по: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Дата обращения: Ссылка активна на 15.06.2025
17. Zakharov AV, Yremeev VV, Chumovatov NV, et al. Clinical and genetic associations of polymorphic alleles of the CYP3A4 gene in drug-resistant pulmonary TB patients. CTRI Bulletin. 2024;8(4):17-30 (In Russ). doi: 10.57014/2587-6678-2024-8-4-17-30.
18. Yunusbaeva MM, Borodina LYa, Bilalov FS, et al. Study of the influence of CYP3A5, CYP2B6 and NAT2 gene polymorphism on the effectiveness of treatment of multidrug-resistant tuberculosis. Farmakogenetika i farmakogenomika = Pharmacogenetics and pharmacogenomics. 2020;(2): 26-27 (In Russ.). doi: 10.37489/2588-0527-2020-2-26-27.
19. Wang N, Chen X, Hao Z, et al. Association of ABCG2 polymorphisms with susceptibility to anti-tuberculosis drug-induced hepatotoxicity in the Chinese population. Xenobiotica. 2022 May;52(5):527-533. doi: 10.1080/00498254.2022.2093685.
About the Authors
D. A. IvanovaRussian Federation
Diana A. Ivanova — PhD, Dr. Sci. (Med), Scientific Secretary, TB Doctor, General Practitioner, The Moscow Research and Clinical Center for Tuberculosis Control of the Moscow Government Department of Health; Professor of the Department of Phthisiology FSBEI FRE RMACPE MOH Russia.
Moscow
Competing Interests:
The study had no sponsorship
E. I. Yurovskaya
Russian Federation
Ekaterina I. Yurovskaya — TB doctor at the TB dispensary department of the North-West District branch, The Moscow Research and Clinical Center for Tuberculosis Control of the Moscow Government Department of Health.
Moscow
Competing Interests:
The study had no sponsorship
K. Yu. Galkina
Russian Federation
Ekaterina I. Yurovskaya — PhD, Cand. Sci. (Biol), Leading Researcher at the Department of Laboratory Diagnostics of Tuberculosis and Pathomorphology, The Moscow Research and Clinical Center for Tuberculosis Control of the Moscow Government Department of Health.
Moscow
Competing Interests:
The study had no sponsorship
What is already known on this topic?
The Treatment Challenge: Treating multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis remains a significant challenge, with treatment success rates (54% in Russia) still far below the WHO target (80%).
The Role of Pharmacogenetics: The response to therapy depends on both phenotypic factors (age, comorbidities, etc.) and genetic factors affecting drug metabolism and transport.
Lack of Data for MDR/XDR-TB: While some pharmacogenetic markers are known for drug-susceptible TB (e.g., NAT2 for isoniazid), personalized approaches for modern MDR/XDR-TB regimens are underdeveloped, and relevant biomarkers are largely unstudied.
What does this study add?
Identifies Novel Efficacy Markers: This clinical study identified, for the first time, two specific genetic polymorphisms significantly associated with poor treatment outcomes in MDR/XDR-TB patients:
Homozygous genotype AA in the CYP3A5 gene (rs776746).
Homozygous genotype AA in the ABCG2 gene (rs2231142).
Identifies Safety/Toxicity Markers: Discovered polymorphisms associated with the risk of specific adverse drug reactions (ADRs):
Neurotoxicity: associated with the wild-type GG genotype in the ABCB1 gene (rs2032582).
Gastrointestinal reactions: associated with the homozygous TT genotype in the ABCB1 gene (rs1128503).
Protective Effect: A polymorphism in the SLCO1B1 gene (rs4149056) was found to reduce the risk of arthralgia.
Proposes a Pharmacogenetic Panel: Defined a specific set of genes (CYP3A5, ABCG2, ABCB1, SLCO1B1) whose analysis could be useful for predicting treatment outcomes.
How might this influence clinical practice in the foreseeable future?
Therapy Personalization: If confirmed in larger studies, genetic testing could allow clinicians to identify patients at high risk of treatment failure (carriers of CYP3A5 AA and ABCG2 AA) before starting therapy.
Tailored Treatment Strategies: For these high-risk patients, longer or intensified chemotherapy regimens could be planned from the outset to improve the chances of success.
Adverse Reaction Prevention: Identifying patients with a genetic risk for specific ADRs (e.g., neurotoxicity) would enable targeted monitoring and allow for proactive dose adjustments or preventive measures, improving treatment tolerability.
Guideline Development: This work lays the foundation for developing clinical guidelines on the use of pharmacogenetic testing in patients with MDR/XDR-TB.
Review
For citations:
Ivanova D.A., Yurovskaya E.I., Galkina K.Yu. Pharmacogenetic markers in the treatment of patients with multidrug-resistant tuberculosis. Pharmacogenetics and Pharmacogenomics. 2025;(2):23-29. (In Russ.) https://doi.org/10.37489/2588-0527-2025-2-23-29. EDN: UISFIM










































