Scroll to:
Correlation between CYP2C9 polymorphisms and office blood pressure levels in patients treated with irbesartan and valsartan
https://doi.org/10.37489/2588-0527-2025-1-24-35
EDN: FEQVXQ
Abstract
Arterial hypertension (AH) is one of the most significant modifiable risk factors for cardiovascular diseases, affecting approximately 1.5 billion people worldwide. The study of genetic polymorphisms involved in blood pressure regulation is a promising direction for elucidating the molecular and biological mechanisms underlying the pathogenesis of hypertension. Analyzing associations between gene variants and the response to antihypertensive therapy offers opportunities to develop personalized treatment strategies aimed at improving the efficacy and safety of pharmacotherapy.
Objective. To evaluate the pharmacodynamic efficacy of angiotensin II receptor blockers (ARBs), used as monotherapy or in combination with hydrochlorothiazide, in patients with newly diagnosed AH depending on their genetic background, specifically the CYP2C9 gene polymorphisms Arg144Cys
(rs1799853, CYP2C9*2) and Ile359Leu (rs1057910, CYP2C9*3).
Materials and methods. The study included 179 patients from the Moscow region with newly diagnosed grade 1–2 arterial hypertension, comprising 141 (78.8 %) women and 38 (21.2 %) men aged 32 to 69 years. Participants were randomly assigned to receive either irbesartan or valsartan as monotherapy or in combination with hydrochlorothiazide using simple randomization. Venous blood samples for genotyping CYP2C9*2 and *3 polymorphisms were collected three weeks after enrollment. Office blood pressure was measured at baseline, at 3 weeks, and at 3 months of therapy.
Results. In patients with newly diagnosed AH who had not previously received antihypertensive treatment, a comparative analysis of the effectiveness of irbesartan and valsartan was performed based on CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu) genotypes. Carriers of the *2 and *3 alleles showed a more pronounced reduction in office systolic and diastolic blood pressure after three weeks of therapy with both irbesartan and valsartan. However, by the end of the 3-month follow-up, no statistically significant association was observed between genotype and the magnitude of the antihypertensive response. The influence of CYP2C9 polymorphisms on heart rate was limited and mostly did not reach statistical significance.
Conclusion. The findings suggest a potential role for pharmacogenetic testing in the initiation of angiotensin II receptor blocker therapy in patients with newly diagnosed arterial hypertension.
Keywords
For citations:
Rebrova E.V., Shikh E.V. Correlation between CYP2C9 polymorphisms and office blood pressure levels in patients treated with irbesartan and valsartan. Pharmacogenetics and Pharmacogenomics. 2025;(1):24-35. (In Russ.) https://doi.org/10.37489/2588-0527-2025-1-24-35. EDN: FEQVXQ
Introduction
According to the World Health Organization (WHO) [1], approximately 50% of adults with hypertension remain undiagnosed. By 2030, approximately 40% of the adult population in the United States is projected to have some form of cardiovascular disease, including hypertension [2]. In this regard, the WHO has identified a 25% reduction in the prevalence of hypertension by 2025 as a global priority [3]. High blood pressure is the leading cause of disability-adjusted life years (DALYs) lost [4] and is responsible for a significant proportion of cardiovascular events [5] and premature mortality [6] worldwide. Despite the obvious importance of prevention in limiting the growth in the prevalence of hypertension and associated comorbid conditions, most clinical guidelines continue to focus primarily on the treatment of the disease [7, 8]. One of the promising areas for increasing the effectiveness of pharmacotherapy for hypertension in patients who regularly take antihypertensive drugs in accordance with current clinical guidelines but do not achieve target blood pressure levels is the introduction of personalized medicine principles. Historically, the idea of the need for an individual approach to treatment has been developing in medicine for centuries, but only in recent decades has the approach to individualization of therapy been based on objective molecular genetic data. The modern concept of personalized medicine involves the use of scientifically based methods for selecting the most effective and safe pharmacotherapy taking into account the individual characteristics of the patient, including genetic markers that affect the pharmacokinetics and pharmacodynamics of drugs. The completion of the Human Genome Project, the active development and clinical implementation of molecular genetic technologies, as well as the accumulation of knowledge about the molecular mechanisms of drug action have contributed to the transition from an empirical to an evidence-based approach in choosing therapy. Given the growing demands for the effectiveness and safety of medical care, the use of pharmacogenetic data is becoming increasingly important in the treatment of chronic diseases, including arterial hypertension [9].
Single nucleotide polymorphisms (SNPs) of cytochrome P450 (CYP) enzymes, key participants in the first phase of biotransformation of many drugs, are among the most important genetic determinants of interindividual variability in drug response. Currently, more than 700 allelic variants of CYP enzymes have been described in various populations, many of which significantly affect their catalytic activity and pharmacokinetic parameters of the corresponding drugs. The highly genetically polymorphic CYP2C9 enzyme is involved in the metabolism of approximately 15–18% of all clinically used drugs, including warfarin, glimepiride, diclofenac, carvedilol, torasemide, and angiotensin II receptor blockers (particularly losartan). The most studied variants that differ from the wild-type CYP2C9*1 allele are CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu), although a significantly larger number of allelic variants are currently known. The CYP2C9*2 allele is caused by the substitution of cytosine for thymine at position 430 of the nucleotide sequence, which results in the substitution of arginine for cysteine at position 144 of the amino acid chain and the formation of an enzyme with reduced metabolic activity. The CYP2C9*3 allele is characterized by the substitution of adenine for cytosine at position 1075, which causes the substitution of isoleucine for leucine at amino acid 359, also associated with a decrease in the functional activity of the enzyme [10–12].
Objective
To study the pharmacodynamic parameters of the effectiveness of therapy with angiotensin II receptor blockers as monotherapy and as part of combination drugs in patients with hypertension depending on the genetic characteristics of patients – Arg144Cys, Ile359Leu polymorphisms of the CYP2C9 gene.
Materials and methods
The study included 179 patients with newly diagnosed stage 1–2 hypertension (AH) living in the Moscow region. The study sample was dominated by women — 141 patients (78.8%); there were 38 men (21.2%). The patients' age ranged from 32 to 69 years; the mean age was 58.2±6.4 years, the median age was 60 years (interquartile range: 57–63 years). Inclusion criteria: established diagnosis of stage 1–2 hypertension, age from 18 to 74 years, presence of signed informed voluntary consent to participate in the study [13].
The study was approved by the local ethics committee of the I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University) (protocol No. 05-21 dated March 10, 2021). The study design was an open, randomized, controlled clinical trial. The clinical and instrumental examination program included collecting complaints and anamnesis (including the presence of risk factors and comorbidities), physical examination, biochemical blood test, office measurement of blood pressure (BP), electrocardiography (ECG) to exclude patients with rhythm disturbances and structural heart pathology, and echocardiography. BP was measured on both arms using the Korotkov method after the patient had rested for 10 minutes in a sitting position. The average value of three consecutive measurements taken at one-minute intervals was used for analysis.
For genotyping, venous blood was collected in 4 ml VACUETTE® vacuum tubes (Greiner Bio-One, Austria) (size 13×75 mm) containing K2-EDTA as an anticoagulant. The tubes were gently inverted at least 10 times to ensure uniform mixing of blood with the anticoagulant, then the samples were frozen and stored at -20°C until DNA extraction. Genetic polymorphisms were analyzed by real-time polymerase chain reaction (RT-PCR) using a CFX96 Touch Real-Time PCR Detection System and CFX Manager software (Bio-Rad, USA), as well as commercial reagent kits. Genotyping of CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu) alleles was performed using the RealBest-Genetics Warfarin kit (cat. No. D-3827, Vector-Best, Russia), based on PCR followed by analysis of amplicon melting curves. Statistical analysis and data visualization were performed in the R software environment version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive characteristics of categorical variables are presented as absolute numbers and relative frequencies. Fisher's exact test was used to compare groups by categorical characteristics. The assessment of the compliance of the genotype distribution with the Hardy-Weinberg principle was carried out using the likelihood ratio criterion [13].
Results
All patients included in the study had not previously received regular antihypertensive therapy. After randomization using the simple random allocation method (envelope method), the participants were divided into two groups receiving therapy with irbesartan or valsartan. Angiotensin II receptor blockers (ARBs) - irbesartan and valsartan - were used as monotherapy or in combination with hydrochlorothiazide for three months. In the irbesartan group, 83 patients received the drug at a dose of 150 mg once a day; of these, 32 patients were on monotherapy, and 51 patients - on combination therapy (irbesartan 150 mg + hydrochlorothiazide 12.5 mg). In the valsartan group, 96 patients received the drug at a dose of 80 mg once a day; Monotherapy was used in 8 patients, and a combination regimen (valsartan 80 mg + hydrochlorothiazide 12.5 mg) was used in 88 patients. When target blood pressure (BP) levels were achieved after 3 weeks of therapy (<140/90 mmHg; if well tolerated - <130/80 mmHg, but not <120/70 mmHg), patients continued taking the prescribed therapy regimen for three months. In case of insufficient BP control, therapy was intensified by doubling the dose of irbesartan or valsartan, both as part of monotherapy and as part of a combination regimen [12]. Venous blood sampling for determination of genetic polymorphisms of CYP2C9 rs1799853 (Arg144Cys, CYP2C9*2) and rs1057910 (Ile359Leu, CYP2C9*3) was performed 3 weeks after inclusion in the study. Office blood pressure measurement was performed at each visit: at the time of inclusion in the study (baseline), after 3 weeks and after 3 months of therapy. All patients were determined the genotype of polymorphisms Arg144Cys, Ile359Leu of the CYP2C9 gene. Among the examined patients, 141 patients (78.8%) had the wild homozygous genotype *1/*1 for the CYP2C9*2 locus, 34 patients (19.0%) had the heterozygous genotype *1/*2, and 4 patients (2.2%) had the mutant homozygous genotype *2/*2. The distribution of genotype frequencies for the CYP2C9*3 polymorphism was as follows: the wild homozygous genotype *1/*1 was found in 146 patients (81.6%), and the heterozygous *1/*3 was found in 33 patients (18.4%). The homozygous mutant genotype *3/*3 was not detected. In addition, 2 patients (1.1%) were found to have the compound heterozygous genotype *2/*3 for the CYP2C9 locus [14]. There were no statistically significant deviations of the observed genotype frequencies from the theoretical ones determined by the Hardy-Weinberg equilibrium: the Arg144Cys polymorphic locus of the CYP2C9 gene (χ²=0.62, p=0.43), the Ile359Leu polymorphic locus of the CYP2C9 gene (χ²=0.91, p=0.341) [15].
Tables 1 and 2 present the results of assessing the dynamics of office SBP, DBP, and HR in patients with different genotypes for the Arg144Cys and Ile359Leu polymorphic markers of the CYP2C9 gene in the irbesartan and valsartan patient groups.
Both the irbesartan and valsartan groups of patients showed a statistically significant decrease in office SBP during the observation period. Carriage of the *2 allele in patients receiving irbesartan was statistically significantly associated with a greater reduction in office SBP at the midterm assessment by an average of 8.3 [95% CI: -12.7–-3.8] mmHg, while in patients receiving valsartan, carriage of this allele was associated with a lesser effect of the drug (mean difference - 7.4 [95% CI: 1.6–13.2] mmHg). The CYP2C9 Arg144Cys genotype was not a statistically significant predictor of the effect of irbesartan at the end of the study (p=0.538), among patients receiving valsartan, a statistically significantly lesser reduction in office SBP was noted by an average of 5.2 [95% CI: 0.8–9.7] mmHg (Fig. 1).
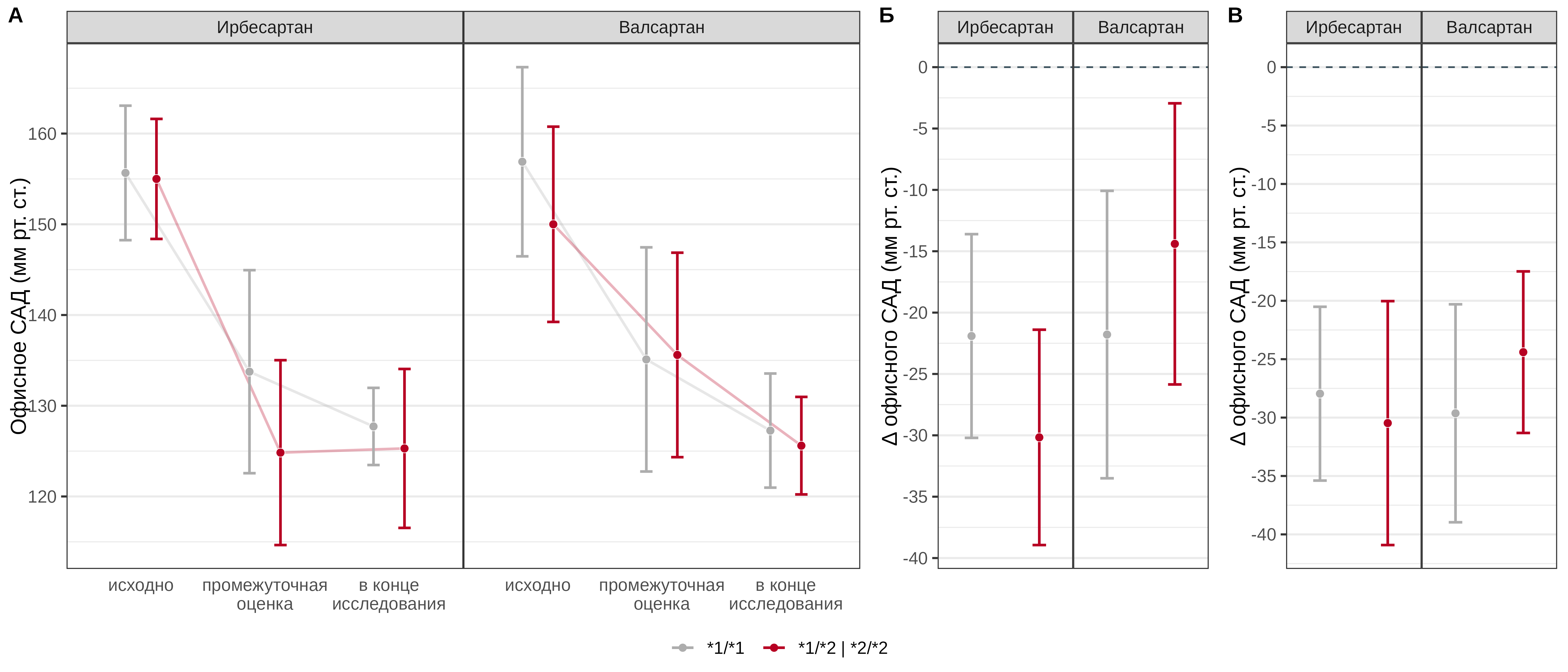
Fig. 1. Comparative analysis of office SBP dynamics in patients with different genotypes of the CYP2C9 Arg144Cys polymorphic marker in the irbesartan and valsartan treatment groups
In both groups, a statistically significant decrease in office DBP was observed throughout the study (p < 0.001). The carriage of the *2 allele in patients receiving irbesartan was statistically significantly associated with a more pronounced decrease in office DBP by an average of 7.5 [95% CI: -11.7–-3.3] mmHg at the interim assessment of the effect; at the end of the study, no statistically significant association of the CYP2C9 Arg144Cys genotype with the effect of irbesartan was found (p = 0.65). In the group of patients receiving valsartan, no statistically significant association of the CYP2C9 Arg144Cys genotype was found either at the interim assessment (p = 0.384) or at the end of the study (p = 0.147) (Fig. 2).
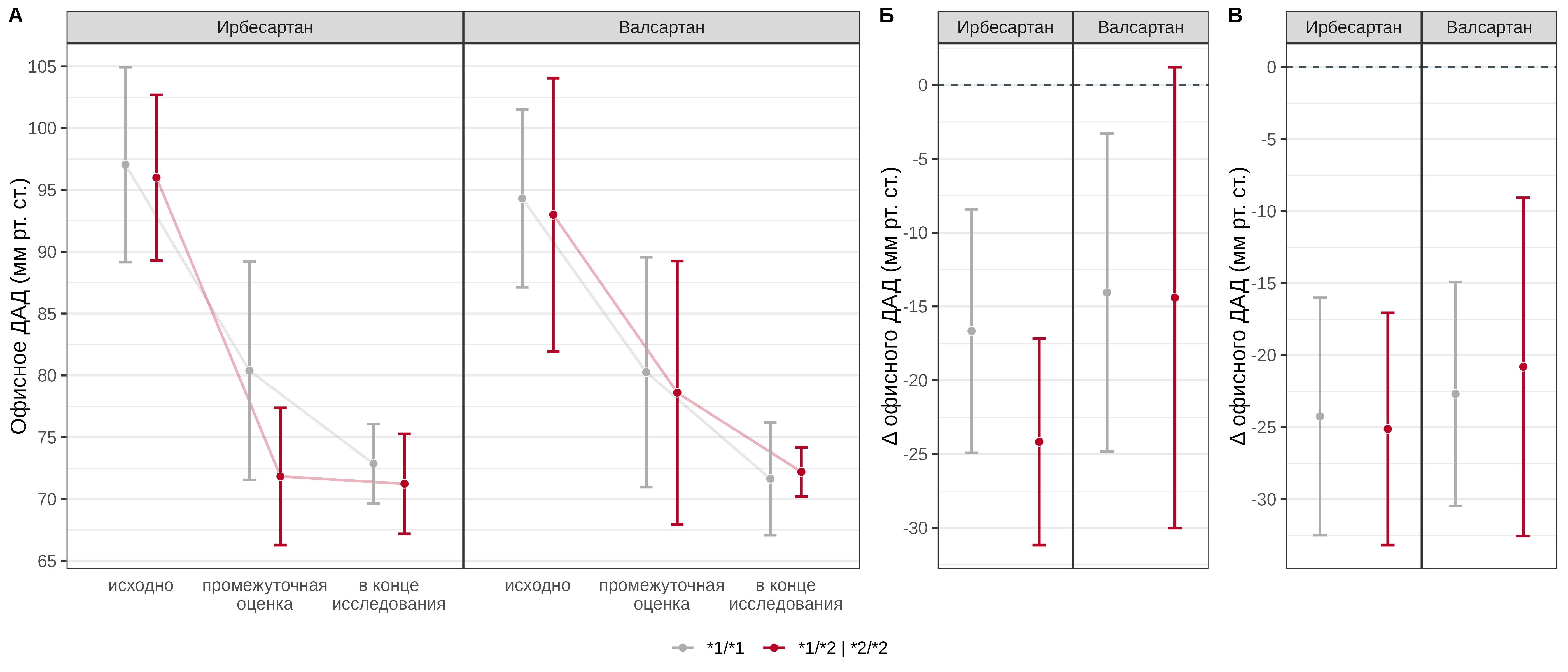
Fig. 2. Comparative analysis of office DBP dynamics in patients with different genotypes of the CYP2C9 Arg144Cys polymorphic marker in the irbesartan and valsartan treatment groups
No statistically significant association of the CYP2C9 Arg144Cys genotype with the effect of irbesartan and valsartan on office HR was found at the interim assessment (p=0.641 and 0.774, respectively). No statistically significant association of the genotype at this locus with the change in office HR at the end of the study compared with baseline values was found in patients receiving irbesartan (p=0.641); in patients receiving valsartan, carriage of the *2 allele was a statistically significant predictor of a more pronounced decrease in HR by an average of 4.4 [95% CI: -6.4–-2.4] beats per minute (Fig. 3).
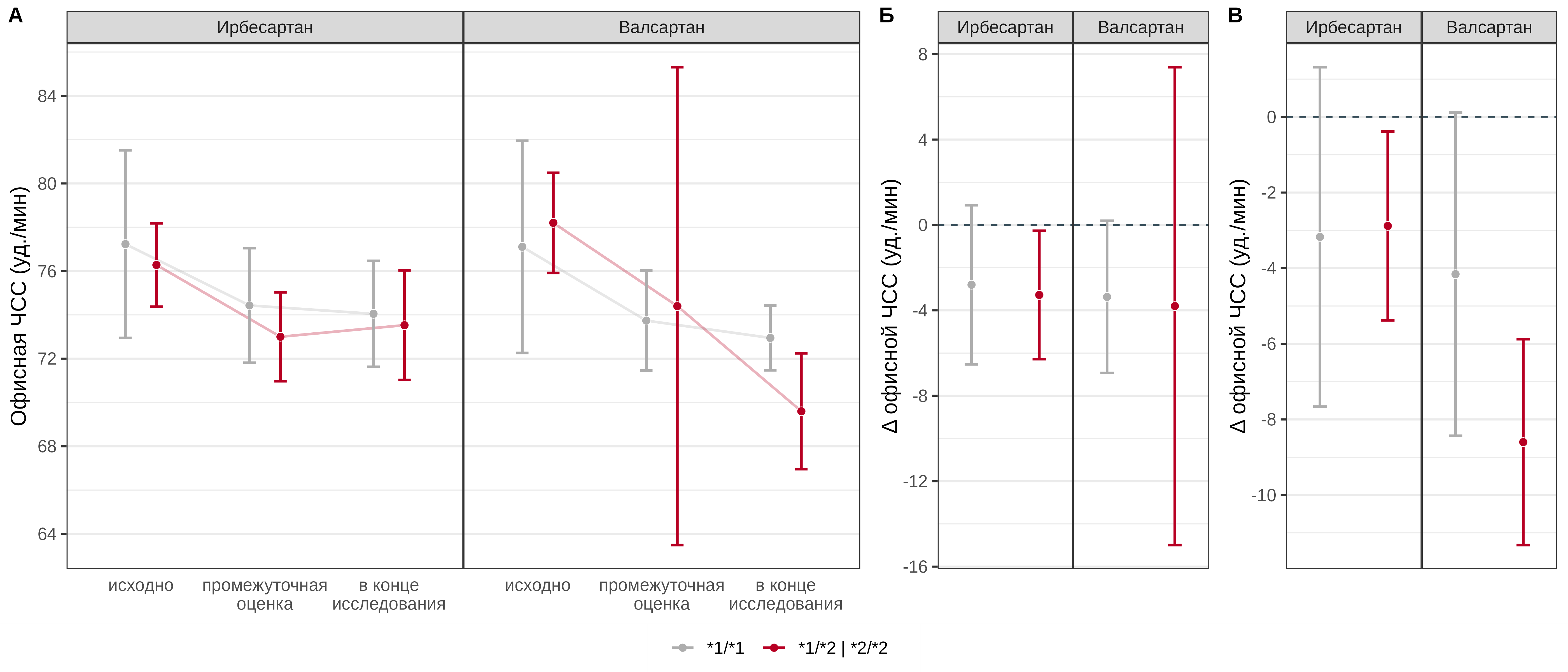
Fig. 3. Comparative analysis of office HR dynamics in patients with different genotypes of the CYP2C9 Arg144Cys polymorphic marker in the irbesartan and valsartan treatment groups
Carriage of the *3 allele in patients receiving irbesartan was statistically significantly associated with a more pronounced reduction in office SBP at the interim assessment by an average of 11.8 [95% CI: -16.6–-7] mmHg, in patients receiving valsartan, carriage of this allele was associated with a more pronounced effect of the drug by an average of 10.5 [95% CI: -16.2–-4.9] mmHg. The CYP2C9 Ile359Leu genotype was not a statistically significant predictor of the effect after 3 months of pharmacotherapy, either with irbesartan (p=0.725) or with valsartan (p=0.146) (Fig. 4).
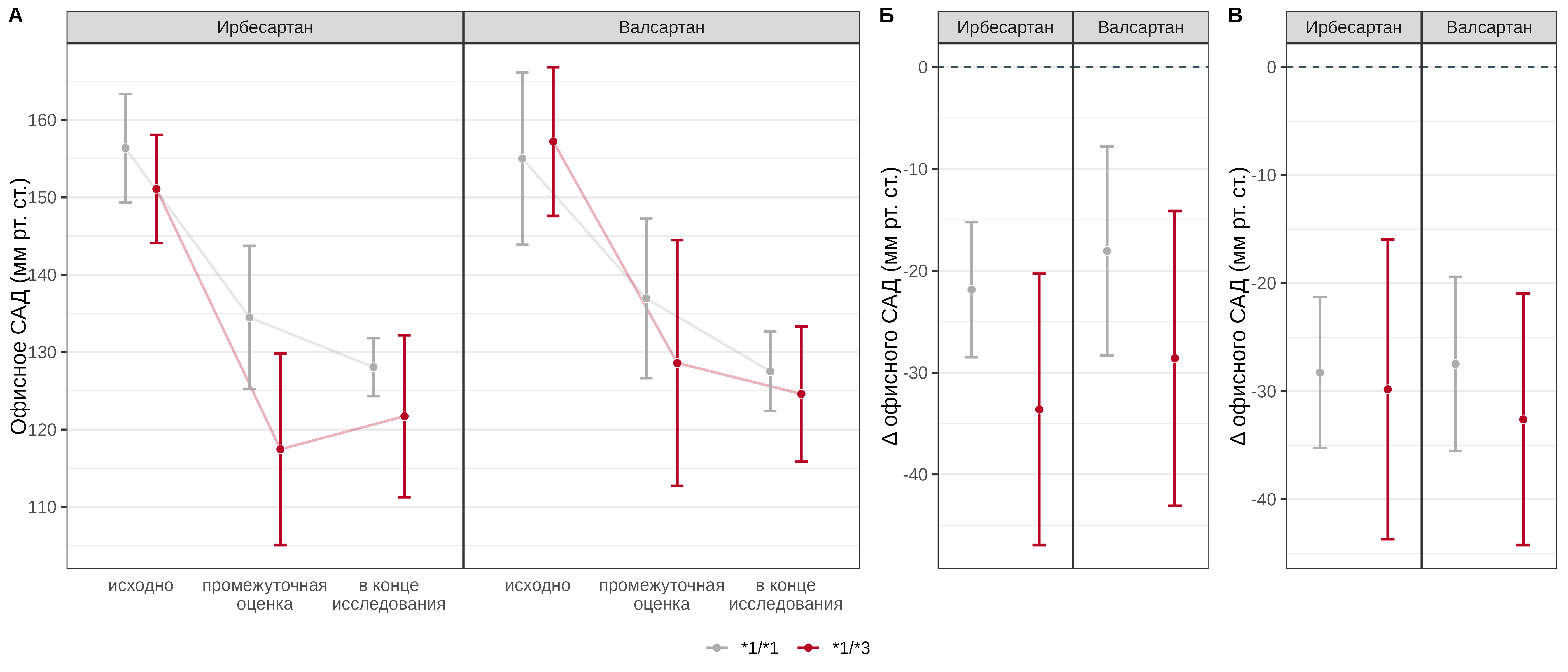
Fig. 4. Comparative analysis of office SBP dynamics in patients with different genotypes of the CYP2C9 Ile359Leu polymorphic marker in the irbesartan and valsartan treatment groups
The carriage of the *3 allele in patients receiving irbesartan was statistically significantly associated with a more pronounced reduction in office DBP at the interim assessment by an average of 7.3 [95% CI: -12.2–-2.4] mmHg, in patients receiving valsartan, the carriage of this allele was also associated with a more pronounced drug effect by an average of 9.7 [95% CI: -15.3–-4.1] mmHg. The CYP2C9 Ile359Leu genotype was not a statistically significant predictor of the effect on office DBP at the end of the study, either with irbesartan (p=0.604) or with valsartan (p=0.119) (Fig. 5).
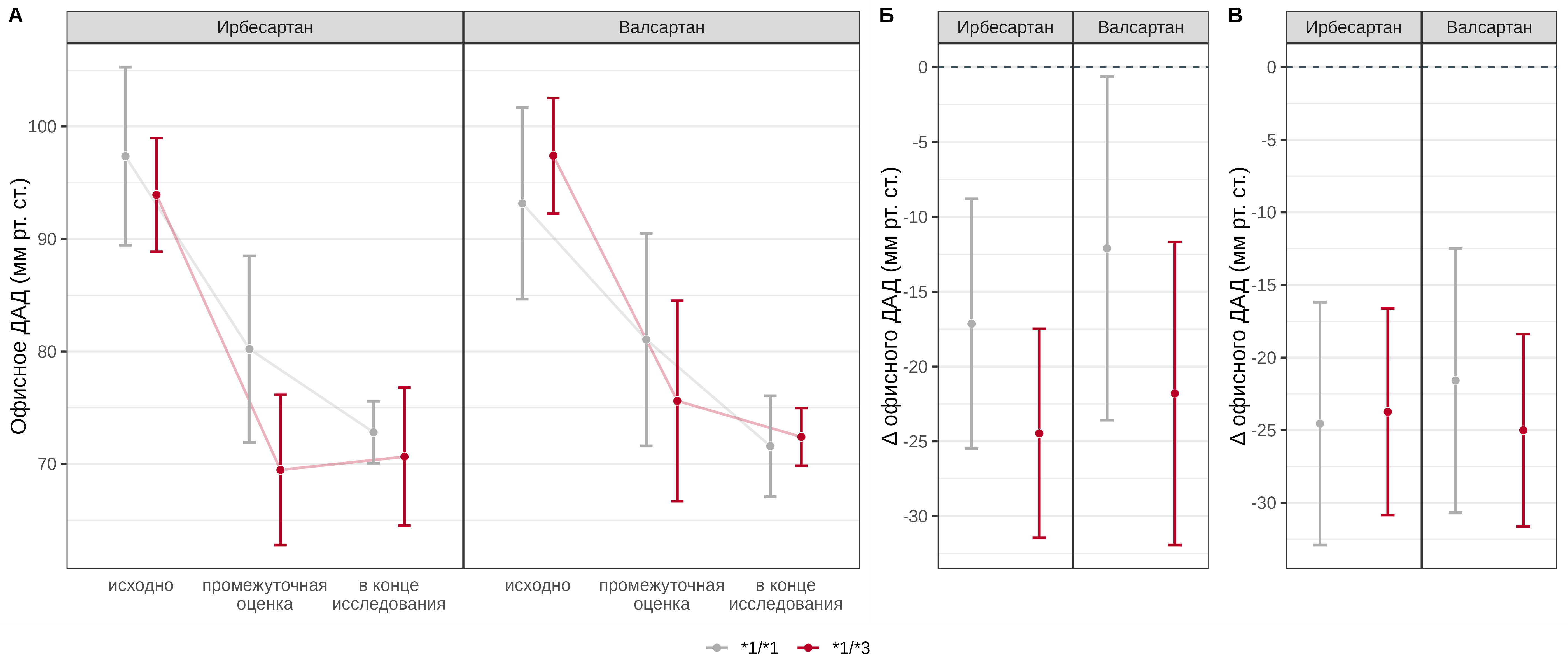
Fig. 5. Comparative analysis of office DBP dynamics in patients with different genotypes of the CYP2C9 Ile359Leu polymorphic marker in the irbesartan and valsartan treatment groups
There was no statistically significant association between the CYP2C9 Ile359Leu genotype and the effect of irbesartan and valsartan on office HR at the interim assessment (p=0.188 and 0.946, respectively). In patients receiving valsartan, carriage of the *3 allele was a statistically significant predictor of a less pronounced decrease in HR by an average of 2.7 [95% CI: 0–5.3] bpm, while the effect was not mediated by steady-state drug concentrations (p=0.766), and there was no statistically significant association between genotype and the change in office HR at the end of the study compared with baseline values in patients receiving irbesartan (p=0.313). It should also be noted that in carriers of the *3 allele who received irbesartan, the change in heart rate was not statistically significant either at the interim assessment or at the end of the study (Fig. 6).
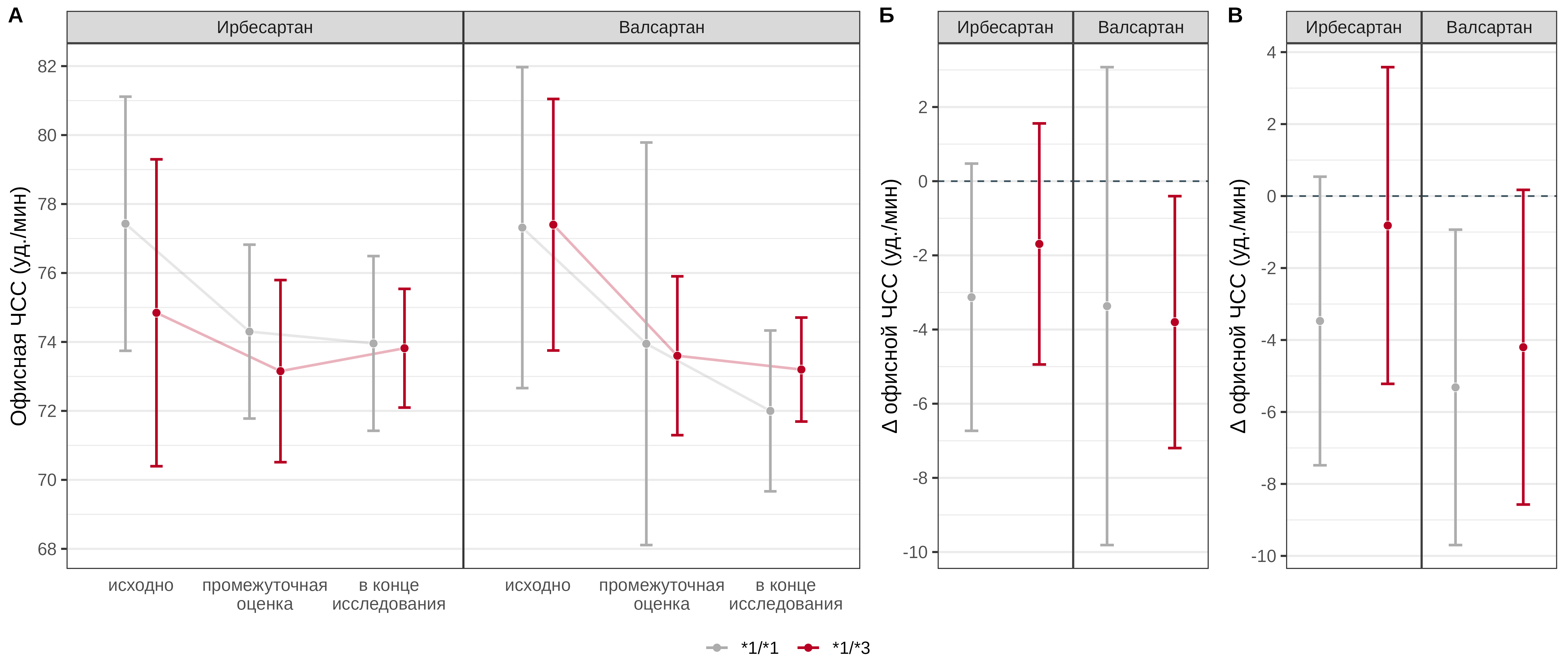
Fig. 6. Comparative analysis of office HR dynamics in patients with different genotypes of the CYP2C9 Ile359Leu polymorphic marker in the irbesartan and valsartan treatment groups
Discussion
Genotypes *1/*2, *2/*2, *1/*3 and *3/*3 for CYP2C9 gene polymorphisms (Arg144Cys, Ile359Leu) are associated with reduced enzyme activity, with homozygous variants showing a marked decrease in its function. In the studied cohort of patients with newly diagnosed grade 1–2 hypertension, the frequency of CYP2C9*2 and CYP2C9*3 risk alleles was 11.7 and 9.2%, respectively, which is comparable to the frequency of these alleles in Europeans — 12 and 6%, respectively. [16]. A meta-analysis published in 2021 [17] pooled data from eight studies conducted up to March 2021 to evaluate the impact of CYP2C9 gene polymorphisms on the pharmacokinetic parameters of losartan and its active metabolite E-3174. In healthy volunteers carrying CYP2C9*2 or CYP2C9*3 alleles, there was a significant increase in the area under the pharmacokinetic curve (AUC) of losartan by 0.17 μg×h/mL (95% CI: 0.04–0.29) compared to carriers of the homozygous wild-type *1/*1. In this case, the AUC of the active metabolite E-3174 was reduced by 0.35 μg×h/mL (95% CI: -0.62 to -0.08), and the maximum concentration (Cmax) was reduced by 0.13 μg/mL (95% CI: -0.17 to -0.09). Also, in carriers of reduced-function alleles, an increase in the half-life was observed for both losartan (+0.47 h; 95% CI: 0.32 to 0.61) and the metabolite E-3174 (+0.68 h; 95% CI: 0.44 to 0.92). These data confirm the clinical significance of CYP2C9 genetic variants in the context of losartan metabolism and, probably, other angiotensin II receptor blockers metabolized with the participation of this enzyme.
According to Sinitsina II et al. [18], polymorphic variants CYP2C9*2 and CYP2C9*3 of the CYP2C9 gene affect the hypotensive effect of losartan in patients with hypertension. The study included 81 patients with newly diagnosed stage 1–2 hypertension, confirmed by 24-hour BP monitoring (ABPM), who were prescribed losartan therapy. Carriage of CYP2C9*2 and CYP2C9*3 alleles was associated with a decrease in the hypotensive effect of the drug (p <0.001; OR=8.13; 95% CI: 2.75–23.97), which was confirmed by significantly higher BP values according to ABPM. At the same time, no significant differences in plasma uric acid levels and the E-3174/losartan ratio in urine were noted between the genotypes. The CYP2C9 isoenzyme plays a key role in the metabolism of not only losartan, but also irbesartan and azilsartan. Unlike losartan, the metabolism of irbesartan and azilsartan via CYP2C9 leads mainly to the formation of inactive metabolites, which causes a potential increase in the concentration of the parent drug in the blood plasma in carriers of alleles with reduced functional activity (CYP2C9*2, CYP2C9*3). This, in turn, may be associated with an increase in the pharmacodynamic effect and an increased risk of adverse drug reactions, which requires a cautious approach to dose selection. In the study by Hallberg P et al. [19], which included 49 patients receiving irbesartan for three months, a more pronounced decrease in diastolic blood pressure was recorded in carriers of the CYP2C9*2 allele: in the group with the CYP2C9*1/*1 genotype (n=33), the decrease was 7.5%, while in patients with the CYP2C9*1/*2 genotype it was 14.4% (p<0.05). This observation is likely associated with increased exposure of irbesartan in individuals with slow metabolism and emphasizes the clinical significance of taking into account CYP2C9 genetic variants when initiating therapy.
Additional data on the effect of CYP2C9 genetic variants on the pharmacokinetics of irbesartan were obtained in a study by Hong X et al. [20], which included 1087 patients of Chinese origin who received irbesartan at a dose of 150 mg once for 28 days. A total of 235 participants were selected for pharmacogenetic analysis. The CYP2C9*2 allele was not detected in the sample, and the frequency of the CYP2C9*3 allele carriage was 3.65%; all carriers had the heterozygous CYP2C9*1/*3 genotype. In this group of patients, the plasma concentration of irbesartan both after 24 hours (on the 27th day of therapy) and 6 hours after administration (on the 28th day) was statistically significantly higher compared to patients with the CYP2C9*1/*1 genotype. Despite the identified pharmacokinetic differences, no significant effect of the CYP2C9 genotype on the degree of blood pressure reduction was found in the study. These results highlight the complex nature of the interaction between pharmacokinetics and clinical response, as well as the need to take into account additional factors when interpreting pharmacogenetic data. In a subsequent analysis performed by Chen G et al. [21], 196 patients who demonstrated a pronounced maximal or minimal hypotensive response to irbesartan therapy were selected from the total number of participants in the Hong X et al. [20] study (n=1087). The frequency of the CYP2C9*3 allele in this subsample was 2.3%. According to the obtained data, in patients with heterozygous CYP2C9*1/*3 genotype, the concentration of irbesartan in blood plasma 6 hours after taking the drug, as well as the degree of reduction in diastolic blood pressure, were statistically significantly higher compared to carriers of the wild type (CYP2C9*1/*1). These results confirm the possible clinical significance of CYP2C9 genotyping in predicting the effectiveness of irbesartan therapy, especially at the initial stages of treatment.
In the study by Choi CI et al. [22], a comparative assessment of the pharmacokinetic parameters of irbesartan was performed in 28 healthy Korean volunteers with different CYP2C9 genotypes: CYP2C9*1/*1 (n=12), CYP2C9*1/*3 (n=10) and CYP2C9*1/*13 (n=6). In carriers of the CYP2C9*3 and CYP2C9*13 alleles, the maximum plasma concentration of irbesartan was significantly higher - by 1.56 and 1.5 times, respectively, the half-life of the drug was increased - by 1.64 and 1.79 times, and oral clearance was significantly lower (by 19.3 and 44.0%) compared to carriers of the "wild" type (CYP2C9*1/*1). The obtained data confirm that a decrease in the metabolic activity of the CYP2C9 enzyme in carriers of reduced-functional alleles can lead to an increase in the systemic exposure of irbesartan, which potentially affects both its efficacy and the safety of therapy. In the study by Dong H et al. [23], 598 patients with hypertension received irbesartan at a dose of 150 mg once a day for 4 weeks. In carriers of the CYP2C9*1/*3 and CYP2C9*3/*3 genotypes, a more pronounced decrease in SBP and DBP (by 34.9±15.5 versus 29.3±10.2 mmHg and by 22.8±9.0 versus 19.6±8.5 mmHg, respectively) was observed compared with patients with the CYP2C9*1/*1 genotype, which correlates with the results obtained among the studied sample of patients in the irbesartan group. Thus, CYP2C9 gene polymorphisms can have a significant impact on the antihypertensive effect of individual representatives of the angiotensin II receptor blockers (ARB) group. In carriers of reduced-functional CYP2C9 alleles, there is a decrease in the effectiveness of losartan therapy, up to the absence of a pronounced hypotensive response, while when using irbesartan and, probably, azilsartan, there is a tendency to increase the pharmacodynamic effect, accompanied by a potential increase in the risk of developing adverse drug reactions. ARB drugs that are not metabolized with the participation of cytochrome P450 isoenzymes (valsartan, candesartan, telmisartan, eprosartan and olmesartan) demonstrate stable antihypertensive activity independent of CYP2C9 genetic variants.
However, our study revealed an association between CYP2C9 genetic variants and the antihypertensive efficacy of valsartan, which contradicts the data on its predominantly extrahepatic elimination and metabolic independence from cytochrome P450 enzymes. This observation may be due to a number of factors, including individual features of liver enzyme expression and activity, involvement of other P450 isoenzymes in valsartan metabolism in individual patients, as well as the possible influence of CYP2C9 polymorphisms on pharmacodynamic parameters, such as angiotensin II receptor sensitivity or drug transport. In addition, it is possible that CYP2C9 genetic variants may indirectly affect the activity of other metabolic or regulatory pathways involved in the implementation of the hypotensive response. These data require further study using a larger sample and population pharmacokinetic analysis.
Conclusion
The obtained results demonstrate a statistically significant decrease in SBP and DBP in patients with newly diagnosed stage 1-2 hypertension during therapy with irbesartan and valsartan for three months. Carriage of polymorphic alleles *2 (Arg144Cys) and *3 (Ile359Leu) of the CYP2C9 gene is associated with a more pronounced decrease in SBP and DBP at the interim assessment of treatment efficacy (after 3 weeks), however, by the end of the observation period, CYP2C9 genotypes were not statistically significant predictors of the antihypertensive effect. The effect of genotype on HR was limited and depended on the combination of the allele and the drug used: carriage of the *2 allele was associated with a more pronounced decrease in HR in patients receiving valsartan, and the *3 allele - with a less pronounced decrease in HR in the same group. Thus, the results of the study confirm the potential role of CYP2C9 polymorphisms in modifying the early response to ARB therapy, which may be important in personalized selection of antihypertensive drugs.
References
1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021 Sep 11;398(10304):957-980. doi: 10.1016/S0140-6736(21)01330-1.
2. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011 Mar 1;123(8):933-44. doi: 10.1161/CIR.0b013e31820a55f5.
3. Kulkarni S. Hypertension management in 2030: a kaleidoscopic view. J Hum Hypertens. 2021 Sep;35(9):812-817. doi: 10.1038/s41371-020-00438-8.
4. Mensah GA. Epidemiology and global burden of hypertension. ESC CardioMed. 2018:290-297. doi: 10.1093/med/9780198784906.003.0061.
5. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and Control of Hypertension: JACC Health Promotion Series. J Am Coll Cardiol. 2018 Sep 11;72(11):1278-1293. doi: 10.1016/j.jacc.2018.07.008.
6. Campbell NRC, Whelton PK, Orias M, et al. 2022 World Hypertension League, Resolve To Save Lives and International Society of Hypertension dietary sodium (salt) global call to action. J Hum Hypertens. 2023 Jun;37(6):428-437. doi: 10.1038/s41371-022-00690-0.
7. Williams B, Mancia G, Spiering W, et al; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018 Sep 1;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339. Erratum in: Eur Heart J. 2019 Feb 1;40(5):475. doi: 10.1093/eurheartj/ehy686.
8. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020 Jun;75(6):1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
9. Petrov VI, Shishimorov IN, Magnitskaya OV, Tolkatchyov BE. Personalized medicine: evolution of methodology and the problems of practical implementation. Journal of Volgograd State Medical University. 2016;1(57):3-11. (In Russ.)
10. Maekawa K, Adachi M, Matsuzawa Y, et al. Structural Basis of Single-Nucleotide Polymorphisms in Cytochrome P450 2C9. Biochemistry. 2017 Oct 17;56(41):5476-5480. doi: 10.1021/acs.biochem.7b00795.
11. Parikh SJ, Evans CM, Obi JO, et al. Structure of Cytochrome P450 2C9*2 in Complex with Losartan: Insights into the Effect of Genetic Polymorphism. Mol Pharmacol. 2020 Nov;98(5):529-539. doi: 10.1124/molpharm.120.000042.
12. Niinuma Y, Saito T, Takahashi M, et al. Functional characterization of 32 CYP2C9 allelic variants. Pharmacogenomics J. 2014 Apr;14(2):107-14. doi: 10.1038/tpj.2013.22.
13. Rebrova EV, Shikh EV. Effect of insertion/deletion polymorphism of angiotensin-converting enzyme gene on efficacy of antihypertensive therapy with angiotensin II receptor blockers. Pharmacy & Pharmacology. 2023;11(6): 494-508. (In Russ.) doi: 10.19163/2307-9266-2023-11-6-494-508.
14. Rebrova EV, Shikh EV. Analysis of the frequency of occurrence of CYP2C9 gene polymorphisms in patients with newly diagnosed arterial hypertension of I–II degree. Pharmacology & Pharmacotherapy. 2023;3: 14-16. (In Russ.) doi: 10.46393/27132129_2023_3_14.
15. Rebrova EV, Shih EV, Kazakov RE, et al. Analysis of the frequency of CYP2C9, AGTR1, AGT, ACE, CYP11B2 gene Polymorphisms occurrence in patients with newly diagnosed 1–2 degree arterial hypertension. Farmateka. 2023;30(14):78-86. (In Russ.) doi: 10.18565/pharmateca.2023.14.78-86.
16. Nikolaeva TIa, Everstova TE, Chugunova SA, et al. Ethnic features of carrier of combinations of CYP2C9 and VKORC1 genotypes among patients with a cardioembolic ischemic stroke. Consilium Medicum. 2020;(22)2:9-12. (In Russ.) doi: 10.26442/20751753.2020.2.200033.
17. Park YA, Song YB, Yee J, et al. Influence of CYP2C9 Genetic Polymorphisms on the Pharmacokinetics of Losartan and Its Active Metabolite E-3174: A Systematic Review and Meta-Analysis. J Pers Med. 2021 Jun 29;11(7):617. doi: 10.3390/jpm11070617.
18. Sinitsina II, Boyarko AV, Temirbulatov II, et al. CYP2C9 gene polymorphisms influence on antihypertensive effectiveness and hypouricemic effect of losartan among patients with arterial hypertension: an observational study. Drug Metab Pers Ther. 2022 Dec 29;38(2):163-168. doi: 10.1515/dmpt-2022-0115.
19. Hallberg P, Karlsson J, Kurland L, et al. The CYP2C9 genotype predicts the blood pressure response to irbesartan: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA) trial. J Hypertens. 2002 Oct;20(10):2089-93. doi: 10.1097/00004872-200210000-00030.
20. Hong X, Zhang S, Mao G, et al. CYP2C9*3 allelic variant is associated with metabolism of irbesartan in Chinese population. Eur J Clin Pharmacol. 2005 Oct;61(9):627-34. doi: 10.1007/s00228-005-0976-8.
21. Chen G, Jiang S, Mao G, et al. CYP2C9 Ile359Leu polymorphism, plasma irbesartan concentration and acute blood pressure reductions in response to irbesartan treatment in Chinese hypertensive patients. Methods Find Exp Clin Pharmacol. 2006 Jan-Feb;28(1):19-24. doi: 10.1358/mf.2006.28.1.962773.
22. Choi CI, Kim MJ, Chung EK, et al. CYP2C9 3 and 13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol. 2012 Feb;68(2):149-54. doi: 10.1007/s00228-011-1098-0.
23. Dong H, Wang FZ, Shi K, et al. Association of Cytochrome P450 2C9*3 and Angiotensin II Receptor 1 (1166A>C) Gene Polymorphisms With the Antihypertensive Effect of Irbesartan. Am J Hypertens. 2021 Feb 18;34(1):121. doi: 10.1093/ajh/hpaa134.
About the Authors
E. V. RebrovaRussian Federation
Ekaterina V. Rebrova, PhD, Cand. Sci. (Med), Associate
Professor
Department of Clinical Pharmacology and Propaedeutics of Internal Diseases
Moscow
Competing Interests:
The authors declare no conflict of interest
E. V. Shikh
Russian Federation
Evgeniya V. Shikh, PhD, Dr. Sci (Med.), Professor, Head of the Department
Department of Clinical Pharmacology and Propaedeutics of
Internal Diseases
Moscow
Competing Interests:
The authors declare no conflict of interest
What is already known on this topic?
- Arterial hypertension (AH) is a global problem and a major modifiable risk factor for cardiovascular diseases.
- Genetic variants (polymorphisms) of the CYP2C9 gene (specifically, *2 and *3) significantly affect the metabolism of many drugs by reducing enzyme activity.
- It is known that these polymorphisms affect the pharmacokinetics and efficacy of losartan (another ARB): in carriers of "slow" alleles, its effect is diminished.
- For irbesartan, data were conflicting: some studies showed higher plasma concentrations in carriers of the *3 allele, but this did not always clearly translate into a greater clinical effect.
- Valsartan is considered metabolically independent of the CYP450 system, and no significant influence of CYP2C9 genetics on its efficacy had been previously described.
What does the new study add?
- Direct comparison of two ARBs: This is the first study to directly compare the influence of CYP2C9 polymorphisms on the efficacy of irbesartan and valsartan within a single trial.
- Identification of a short-term effect: The study shows that carrying the *2 and *3 alleles is associated with a more pronounced reduction in blood pressure (both SBP and DBP) after just 3 weeks of therapy with both drugs.
- New data on valsartan: Surprisingly, the study found that the efficacy of valsartan (which is barely metabolized by CYP2C9) is also influenced by these genetic variants, especially in the short term. This is a novel finding that contradicts established views.
- Effect "leveling off" by 3 months: By the end of the 3-month treatment course, no statistically significant difference in efficacy between carriers of different genotypes remained. This suggests that the influence of genetics is most important at the start of treatment.
How might this affect clinical practice in the foreseeable future?
- Rationale for pharmacogenetic testing at therapy initiation: The results indicate the potential benefit of genetic testing (CYP2C9) at the very beginning of AH therapy selection to predict the early treatment response.
- Personalized drug and dose selection: Knowing a patient's genotype could help a physician:
- For irbesartan: In carriers of "slow" alleles, a more powerful initial effect can be expected, potentially allowing for earlier achievement of target BP and less frequent need for therapy intensification (dose increase).
- For valsartan: The discovered association requires further study, but if confirmed, it would also open possibilities for more accurate response prediction.
- Improving treatment adherence: Rapid achievement of target BP levels in the first weeks of treatment can motivate the patient and increase their long-term adherence to therapy.
- Need for further research: For implementation into routine practice, larger-scale studies and an assessment of the cost-effectiveness of this approach are necessary.
Review
For citations:
Rebrova E.V., Shikh E.V. Correlation between CYP2C9 polymorphisms and office blood pressure levels in patients treated with irbesartan and valsartan. Pharmacogenetics and Pharmacogenomics. 2025;(1):24-35. (In Russ.) https://doi.org/10.37489/2588-0527-2025-1-24-35. EDN: FEQVXQ











































