Scroll to:
Optimizing tamoxifen therapy: the importance of pharmacogenetic testing to improve adherence
https://doi.org/10.37489/2588-0527-2025-1-36-40
EDN: CJHUHQ
Abstract
Tamoxifen is the gold standard of endocrine therapy in patients with ER+ breast cancer. However, the efficacy of tamoxifen directly depends on adherence to treatment. Adherence may decrease due to adverse drug reactions and individual differences in drug metabolism. The article presents data from a study of adherence to tamoxifen therapy after 5 years of follow-up in relation to pharmacogenetic associations and adverse drug reactions. Pharmacogenetic testing can improve adherence to treatment by personalizing therapy and reducing the incidence of adverse drug events.
For citations:
Golubenko E.O., Savelyeva M.I., Korennaya V.V. Optimizing tamoxifen therapy: the importance of pharmacogenetic testing to improve adherence. Pharmacogenetics and Pharmacogenomics. 2025;(1):36-40. (In Russ.) https://doi.org/10.37489/2588-0527-2025-1-36-40. EDN: CJHUHQ
Introduction
Tamoxifen remains the gold standard of endocrine therapy in patients with estrogen receptor-positive breast cancer (BR). However, its effectiveness directly depends on treatment adherence, which is often reduced by adverse drug reactions and individual differences in drug metabolism. Pharmacogenetic studies can predict the response to tamoxifen therapy and optimize treatment.
Adherence to long-term (5-10 years) tamoxifen therapy remains insufficient: according to studies, up to 50% of patients reduce the dose or stop treatment. The main reasons:
- Adverse drug reactions (ADRs): hot flashes, tamoxifen-induced endometrial alterations, bone and joint pain, asthenia, thromboembolism [1].
- Psychological factors (depression, anxiety, forgetfulness) [2].
- Lack of awareness of the importance of long-term use.
Tamoxifen is a prodrug, its active metabolite - endoxifen - is formed under the action of the CYP2D6 enzyme. Polymorphisms of the CYP2D6 gene can significantly affect the concentration of endoxifen. Ultra-fast metabolizers are characterized by high levels of endoxifen, a higher risk of developing ADRs, and intermediate and slow metabolizers - reduced efficacy, a higher risk of relapse [3]. In addition to CYP2D6, polymorphic variants of the CYP3A5*3, CYP2C9*2, CYP2C9*3, CYP2C19*2, CYP2C19*3 and ABCB1 genes play an important role.
Over the course of five years, from 2017 to 2022, we conducted a cohort clinical study, which included three stages. The initial stage, implemented in 2017, was a retrospective analysis of clinical and epidemiological data to determine the incidence and risk factors for the development of endometrial hyperplasia in patients with breast cancer registered in oncological institutions in Moscow (n = 230) [18].
The second stage was devoted to a pharmacogenetic study aimed at studying the clinical manifestations of ADRs of endocrine therapy with tamoxifen and assessing the correlation between the presence of polymorphic variants of genes encoding cytochrome P-450 enzymes and drug transporter proteins and the occurrence of ADRs in patients with breast cancer. This stage involved 120 women with breast cancer, who underwent genetic testing of polymorphic variants of the genes encoding cytochrome P-450 enzymes and P-glycoprotein (Pg). Association analysis revealed a relationship between these polymorphisms and the development of adverse drug reactions to tamoxifen, indicating the clinical significance of genetic polymorphisms of CYP2D6, CYP3A5, CYP2C9 and ABCB1 [4, 5]. We then examined adherence to tamoxifen therapy after 5 years of observation in relation to the previously identified pharmacogenetic associations.
Material and methods
This questionnaire-based study involved 54 patients with breast cancer who had previously undergone a pharmacogenetic study. Data were collected via telephone interviews or electronic questionnaires. The proposed questionnaire included questions regarding the frequency of visits to an oncologist and gynecologist during the five-year period of taking tamoxifen. Due to the limited sample size, the delta percentage (∆%) method was used to analyze the differences identified, with a significance threshold of 5%. The previously identified results of the pharmacogenetic study were taken into account when interpreting the data obtained during this survey study [4, 5].
Results and discussion
The study involved 54 patients, which is 45% of the total number of respondents. Disease progression was recorded in 9.26% of the participants (n=5) (of this subgroup, 4 patients spontaneously discontinued tamoxifen, one patient strictly followed the therapy regimen); while the disease did not progress in 90.74% (Fig. 1). The majority of respondents, namely 59.26% (n=32), continue tamoxifen therapy at the previous dosage and without interruption. The remaining 40.74% (n=22) stopped taking tamoxifen. In this group, 9 patients switched to alternative treatment using aromatase inhibitors, and 13 independently stopped taking the drug due to intolerance. With the exception of tamoxifen-induced endometrial alterations, the incidence of drug-related adverse events was higher in the group of patients who discontinued tamoxifen (Table 1).
Table 1
Comparison of adverse drug reactions of tamoxifen in two groups of patients with breast cancer: those who continued and those who stopped taking the drug
Adverse drug reaction | Continue tamoxifen therapy, n (%) | Stopped taking tamoxifen, n (%) | ∆% |
Tamoxifen-induced endometrial alterations | 7 (21,88 %) | 4 (18,18 %) | 3,7 |
Tides | 21 (65,63 %) | 17 (77,27 %) | -11,64 |
Asthenia | 15 (46,88 %) | 12 (54,55 %) | -7,67 |
Bone pain | 15 (46,88 %) | 12 (54,55 %) | -7,67 |
Dyspepsia | 5 (15,63 %) | 2 (22,22 %) | -6,59 |
Total | 32 (100 %) | 22 (100 %) | 0 |
Over the five-year monitoring period, it was found that 57.4% of the subjects (a total of 31) visited an obstetrician-gynecologist at least once a year, while 42.59% (n=23) sought gynecological care less than once a year.
A significant difference in the frequency of observation by an obstetrician-gynecologist was noted between the groups of patients. In the group of patients continuing tamoxifen therapy, 75% (n=24) were regularly observed by an obstetrician-gynecologist. In contrast, in the group of patients who discontinued tamoxifen, only 31.82% (n=7) observed the regularity of visits. It is noteworthy that in the subgroup of patients who discontinued tamoxifen due to its poor tolerability (n=13), the frequency of observation was the lowest, amounting to 7.69%. Of the thirteen patients (100%) who discontinued tamoxifen therapy on their own due to unfavorable tolerability, ten (76.92%) experienced hot flashes. Seven of them (53.85%) had the GG genotype of the CYP2D6*4 polymorphic variant. Asthenia was recorded in eight participants (61.54%), and seven of them (53.85%) had the homozygous reference genotype 42614AA for rs1057910 of the CYP2C9 gene. In this subgroup, endometrial hyperplasia during treatment was diagnosed in four women (30.77%), and the TT genotype of the ABCB1 3435T>C polymorphic variant was detected in only one patient (7.69%). Seven patients (53.85%) complained of bone pain; Four of them (30.77%) had the GG genotype of the CYP2D6*4 polymorphic variant, the same number (30.77%) had the CT genotype of the ABCB1 3435T>C polymorphic variant, and all patients in this group (53.85%) had the AA genotype of the CYP2C9*3 polymorphic variant. According to the data obtained, these polymorphic variants demonstrate a statistically significant association with the occurrence of bone pain. Dyspeptic phenomena were observed in six patients (46.15%), with four (30.77%) having the TT genotype of the ABCB1 3435T>C polymorphic variant (Fig. 1).
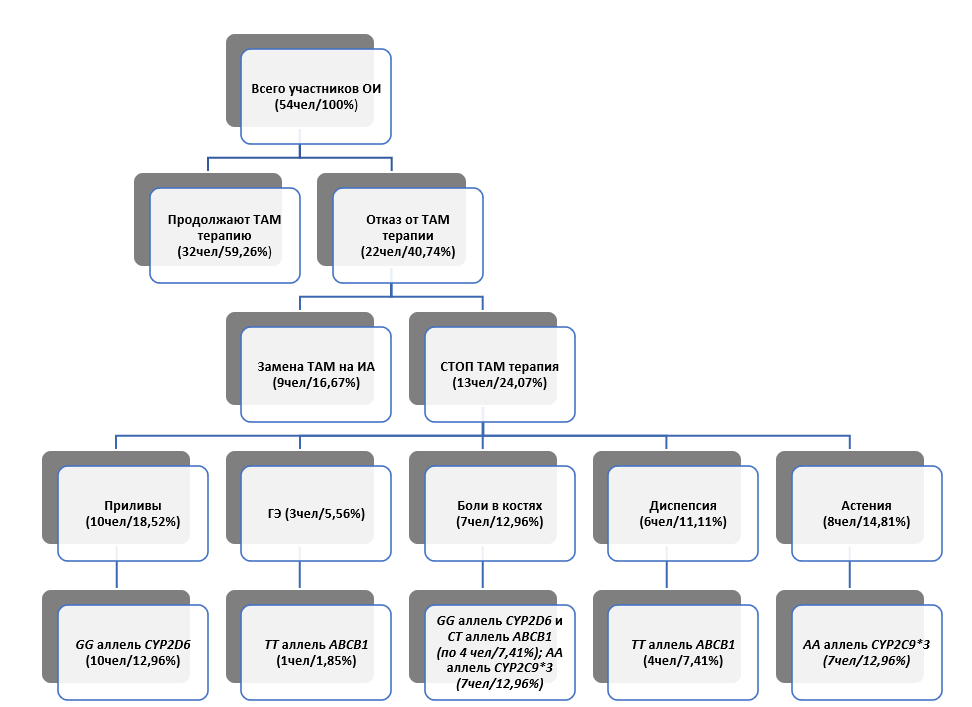
Fig. 1. Results of the study of adherence in relation to the presence of polymorphic variants of the cytochrome P-450 system genes and the ABCB1 gene
Notes: ОИ — questionnaire survey; ТАМ — tamoxifen; СТОП ТАМ — discontinuation of tamoxifen therapy; ГЭ — endometrial hyperplasia; ИА — aromatase inhibitors.
A pattern can be observed in all analyzed patient groups: more pronounced tamoxifen ADRs occur more often in individuals with certain genetic variations. These polymorphisms affect genes responsible for the synthesis of cytochrome P-450 enzymes and transport proteins involved in drug metabolism.
Conclusion
Clinical application of pharmacogenetics to improve adherence is possible in the following aspects:
- Dose personalization — increasing the dose in slow metabolizers or switching to aromatase inhibitors in patients with a high risk of inefficiency.
- Toxicity prediction — preventing drug discontinuation due to ADRs.
- Patient education — explaining the genetic reasons for an individual response to therapy increases motivation to adhere to the treatment regimen [6].
Thus, pharmacogenetic testing before prescribing tamoxifen can improve treatment adherence by personalizing therapy and reducing the incidence of adverse drug reactions. The introduction of genetic screening into clinical practice requires further research, but is already capable of increasing adherence to breast cancer treatment.
References
1. Drullinsky PR, Hurvitz SA. Mechanistic basis for PI3K inhibitor antitumor activity and adverse reactions in advanced breast cancer. Breast Cancer Res Treat. 2020 Jun;181(2):233-248. doi: 10.1007/s10549-020-05618-1.
2. Kolberg HC, Jackisch C, Hurvitz SA, et al. Is weight-based IV dosing of trastuzumab preferable to SC fixed-dose in some patients? A systematic scoping review. Breast. 2021 Jun;57:95-103. doi: 10.1016/j.breast.2021.03.003.
3. Chan CWH, Law BMH, So WKW, et al. Pharmacogenomics of breast cancer: highlighting CYP2D6 and tamoxifen. J Cancer Res Clin Oncol. 2020 Jun;146(6):1395-1404. doi: 10.1007/s00432-020-03206-w.
4. Golubenko EO, Savelyeva MI, Sozaeva ZhA, et al. Clinical significance of genetic polymorphism of tamoxifen metabolic enzymes and transporters in breast cancer: results of a population-based cohort study. Farmateka. 2022;29(11-12):118-126. (In Russ.) doi: 10.18565/pharmateca.2022.11-12.118-126.
5. Golubenko EO, Savelyeva MI, Sozaeva ZA, et al. Predictive modeling of adverse events of tamoxifen therapy for breast cancer (results of a cohort study). Pharmacogenetics and pharmacogenomics. 2022;(1):63-73. (In Russ.) doi: 10.37489/2588-0527-2022-1-63-73.
6. Bolhuis K, Bond MJG, Van Amerongen MJ, et al; Dutch Colorectal Cancer Group Liver Expert Panel. The role of tumour biological factors in technical anatomical resectability assessment of colorectal liver metastases following induction systemic treatment: An analysis of the Dutch CAIRO5 trial. Eur J Cancer. 2023 Apr;183:49-59. doi: 10.1016/j.ejca.2023.01.013.
About the Authors
E. O. GolubenkoRussian Federation
Ekaterina O. Golubenko, Assistant
Department of Obstetrics and Gynecology
Moscow
Competing Interests:
The authors declare no conflict of interest
M. I. Savelyeva
Russian Federation
Marina I. Savelyeva, PhD, Dr. Sci. (Med.), Professor, Professor of the Department
Department of Therapy named EN Dormidontov
Yaroslavl
Competing Interests:
The authors declare no conflict of interest
V. V. Korennaya
Russian Federation
Vera V. Korennaya, PhD, Cand. Sci. (Med), Associate Professor
Department of Obstetrics and Gynecology
Moscow
Competing Interests:
The authors declare no conflict of interest
Review
For citations:
Golubenko E.O., Savelyeva M.I., Korennaya V.V. Optimizing tamoxifen therapy: the importance of pharmacogenetic testing to improve adherence. Pharmacogenetics and Pharmacogenomics. 2025;(1):36-40. (In Russ.) https://doi.org/10.37489/2588-0527-2025-1-36-40. EDN: CJHUHQ











































