Scroll to:
Pharmacogenetic features of angiotensin-converting enzyme inhibitors
https://doi.org/10.37489/2588-0527-2024-2-19-28
EDN: BXQLYJ
Abstract
Interest in the rational prescription of medicines, considering the genetic characteristics of patients, is increasing every year. In foreign medicine, pharmacogenetic testing is often used as the main tool for selecting individual therapy. The most significant interest has been shown in candidate genes involved in changing the pharmacological response to therapy in patients with cardiovascular diseases because of the high risk of mortality. In most cases, cardiovascular disorders are accompanied by high blood pressure, which can be reduced using ACE inhibitors. However, data on the effectiveness and safety of drug use vary depending on the gender, race, or ethnicity of patients, making it more difficult to develop a unified algorithm for the introduction of pharmacogenetic tests into clinical practice. The authors of this review attempted to systematize the data obtained from various studies and identify the presence of clinically significant correlations between changes in the effectiveness of ACE inhibitors and the presence of polymorphism of candidate genes of the renin-angiotensin-aldosterone system.
For citations:
Kantemirova B.I., Komarova O.V., Romanova A.N. Pharmacogenetic features of angiotensin-converting enzyme inhibitors. Pharmacogenetics and Pharmacogenomics. 2024;(2):19-28. (In Russ.) https://doi.org/10.37489/2588-0527-2024-2-19-28. EDN: BXQLYJ
Introduction
One of the modern trends in Russian medicine is a personalized approach to treating patients, considering their individual genetic and phenotypic characteristics. The trend toward continuous study of factors affecting the effectiveness of therapy allows us to improve the treatment of patients, thereby preventing severe diseases, disability, and death. One of these factors is gene polymorphism, which can change the outcome of the prescribed therapy, both positively and negatively. Drugs may be ineffective as fast metabolizers because accelerated metabolism does not allow the active substance to reach the reference concentration values. For slow metabolizers, incorrect selection of the drug dosage can lead to intoxication of the body due to the accumulation of metabolites. The widespread dissemination of information on the features of the use of pharmacogenetic testing, which allows us to avoid such irrational prescription of drugs, indicates a significant role of genotyping in the clinical practice of treating patients with various pathologies [1].
From the standpoint of evidence-based medicine, personalization in patient treatment plays a leading role, and the use of pharmacogenetic technologies significantly simplifies the tasks of the attending physician in selecting the most effective therapy. Detection of individual characteristics of changes in the pharmacokinetics and pharmacodynamics of drugs in each patient allows influencing the processes of absorption, distribution, biotransformation, excretion and impact on the physiological target in each specific case. Influencing these processes has become possible due to the use of such clinical and pharmacological approaches as: pharmacogenetic testing, therapeutic drug monitoring, patient phenotyping, etc. [2]. Determining the relationship between the polymorphism of genes encoding biotransformation enzymes or drug transporters and the profile of the effectiveness and safety of therapy has the potential to become a single diagnostic standard in the near future [3]. Currently, clinical guidelines are already being adjusted due to several meta-analyses, systematic reviews and randomized clinical trials proving the need for preliminary genotyping of patients of different ethnic groups. The most significant impact of pharmacogenetic testing is noted for treating patients with comorbid and chronic diseases because the treatment is long-term and requires constant monitoring of indicators. Such nosologies include arterial hypertension (AH), for the treatment of which, in most cases, angiotensin-converting enzyme (ACE) inhibitors are used in mono- or combination therapy. In this regard, the purpose of this review was to summarize the data on the role of candidate gene polymorphisms that affect the efficacy and safety of ACE inhibitors.
Methods
The search for data for the review was conducted among domestic and foreign literary sources in the RSCI (elibrary.ru) and Google Academy databases using the following keywords: "gene polymorphism", "ACE inhibitors", "arterial hypertension".
Foreign studies were analyzed in the MEDLINE PubMed and ResearchGate databases using the following keywords: "gene polymorphism", "ACE inhibitors", "arterial hypertension". After excluding matches, 626 publications in English that met the query requirements were found.
The search for clinically significant candidate genes was conducted on the pharmgkb.org platform in the "Clinical Annotations" section.
When selecting the literature, 48 publications were analyzed that best met the inclusion criteria, such as: clinically significant gene polymorphism in patients taking ACE inhibitors as mono- and combination therapy; patients over 18 years of age, with a confirmed diagnosis of hypertension or essential hypertension. The selected publications had the highest number of citations and were also referred to by leading experts in the field of pharmacogenetics.
Results
Patients seeking help at medical institutions or undergoing annual medical examinations in 78% of cases either had already been diagnosed with cardiovascular diseases or complained of high blood pressure or heart pain during the initial examination without an aggravated medical history. According to the World Health Organization (WHO), the number of newly diagnosed cases of hypertension in 2024 exceeded 1 billion cases [4].
According to clinical guidelines, the treatment of hypertension is based on the use of a group of ACE inhibitors as mono- and combination therapy (ACE inhibitors + calcium channel blockers; ACE inhibitors + diuretic). This group is most often prescribed to patients because it has a complex effect on the renin-angiotensin-aldosterone system (RAAS), blocking the production of angiotensin II (Fig. 1).
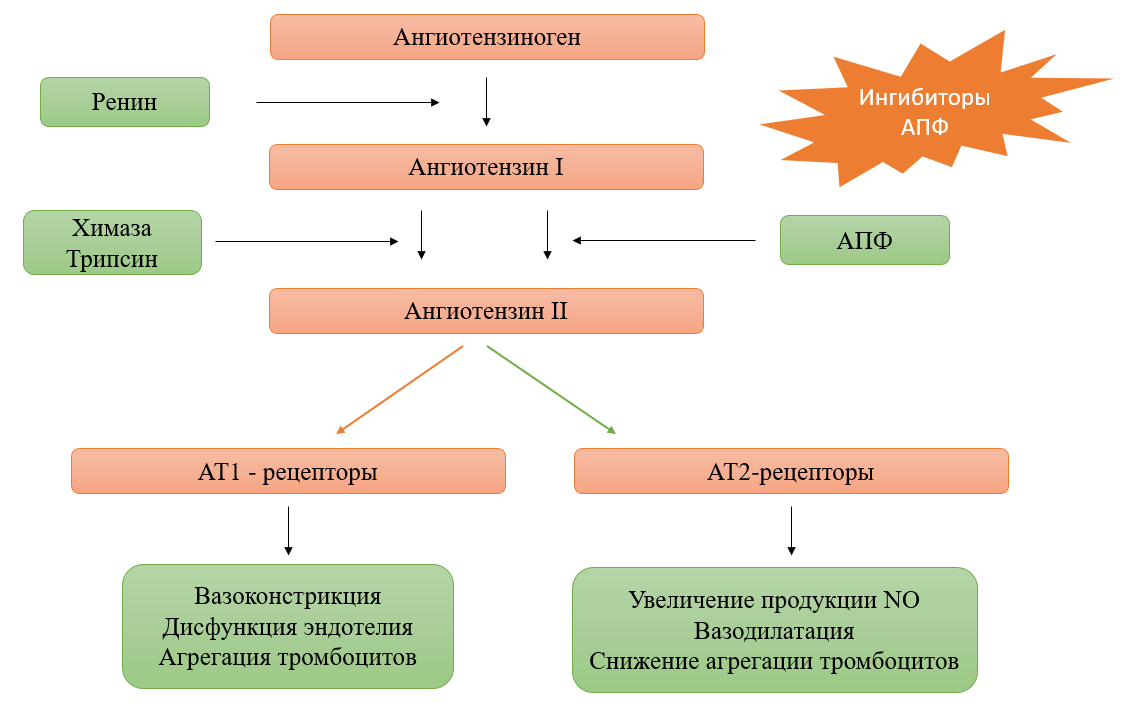
Fig. 1. Structural components of the renin-angiotensin system and related pharmacological responses to ACE inhibitors
Patients receiving long-term antihypertensive therapy with ACE inhibitors note various side effects, as well as in some cases allergic reactions and changes in blood biochemical parameters. Having noticed the difference between the clinical effect and the frequency of adverse reactions (AR), it was concluded that the carriage of polymorphic genotypes in patients has a significant impact on therapy. These features that affect the pharmacological response are single-nucleotide polymorphisms of genes (SNP), which require mandatory identification and consideration when adjusting therapy.
The effectiveness of ACE inhibitors depends on numerous candidate genes involved in the pharmacodynamics and pharmacokinetics of drugs that affect the RAAS (Fig. 2).
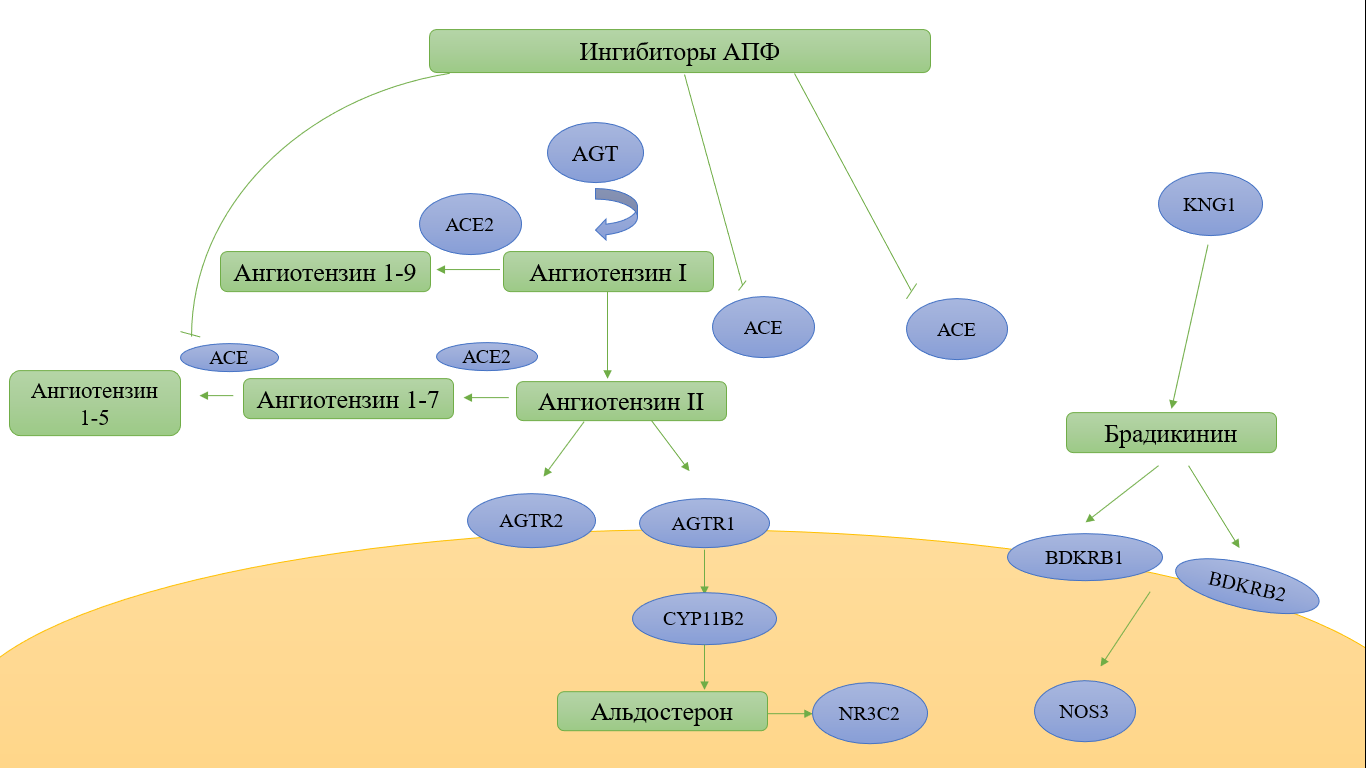
Fig. 2. Candidate genes affecting the effectiveness of ACE inhibitors
According to the resource pharmgkb.org, there are several genes, SNP in which can lead to a change in the efficacy and safety of drugs in the study group. These genes include: ACE, ACE2, AGT, AGTR1, BDKRB1, NR3C2, MTHFR, ABO, PRCP, CYP11B2, CES1, and the solute transporter gene-SLCO1B1. SNPs in the presented candidate genes are located in different loci and participate in the regulation of blood pressure (BP), water-electrolyte balance and the work of the sympathetic nervous system (Fig. 3).
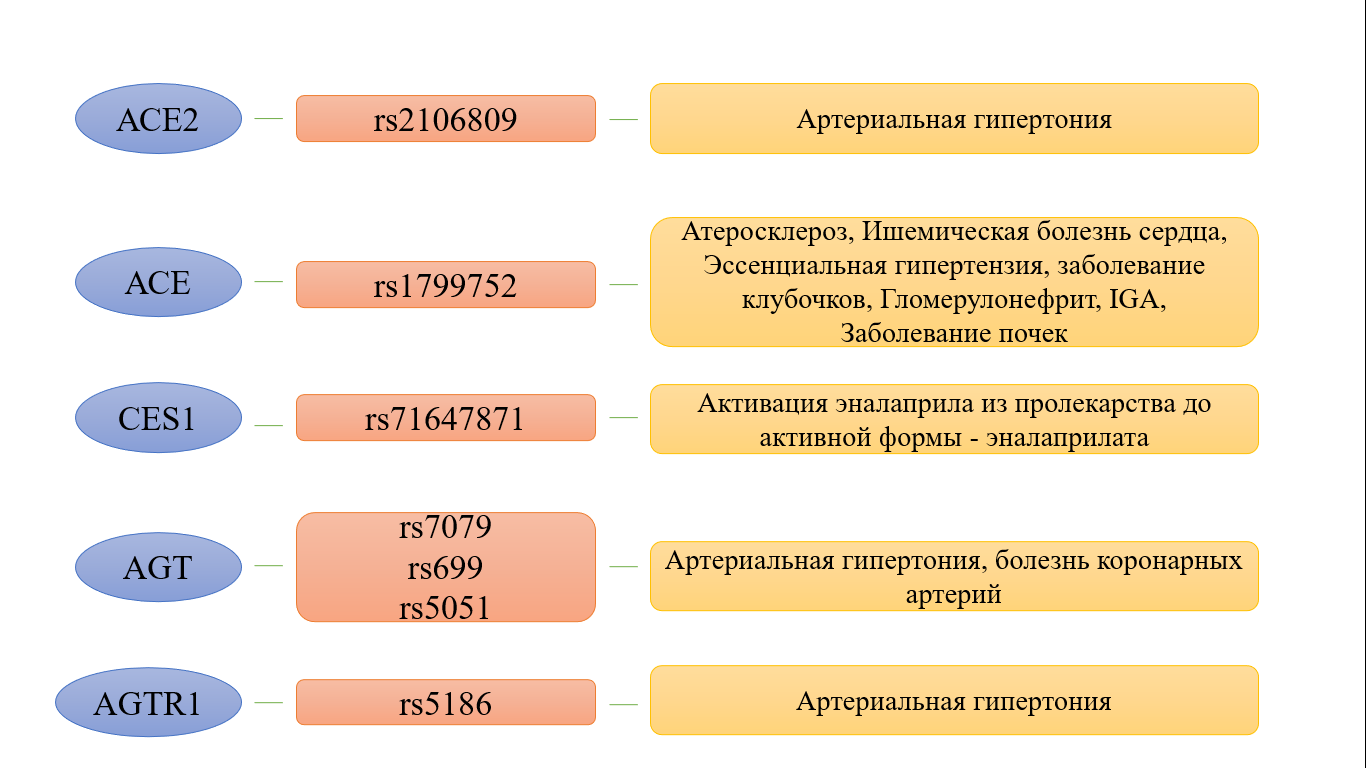
Fig. 3. SNPs and phenotypes of the most studied candidate genes
ACE2. The ACE2 gene, for which more than 180 nucleotide sequence variants are currently known, encodes the membrane protein angiotensin-converting enzyme 2, which catalyzes the conversion of angiotensin I to angiotensin 1-9 and angiotensin II to angiotensin 1-7. The presented protein is a functional cellular receptor for penetrating the SARS-CoV-2 virus, which affects its virulence. However, at the moment, it has not been scientifically proven that the use of ACE inhibitors can lead to a fatal outcome in patients with cardiovascular pathologies and COVID-19, despite an increase in the expression of angiotensin-converting protein ACE2 mRNA. The clinical benefits of using this group of drugs are much greater than the harm from their use [5]. According to the literature, the rs2106809 polymorphism is the most studied and clinically significant. It was noted that in women with the AA and AG genotype, compared with the GG genotype, there was the most significant decrease in diastolic blood pressure (DBP) from taking benazepril and imidapril (9.62 ± 6.83 or 10.2 ± 7.2 versus 6.81 ± 6.31 mm Hg, respectively; p = 0.045) [6]. In a case-control study of the rs2106809 polymorphism, it was noted that in patients with the CC genotype, the diastolic blood pressure reached the reference values faster when taking captopril than in the CT + TT genotypes. This study confirmed the hypothesis that patients with the CC genotype manage to achieve normalization of blood pressure figures faster than carriers of the TT genotype [7]. Large-scale studies indicate not only the relationship between changes in drug activity and the carriage of gene polymorphisms but also that SNPs in some cases are predictors of the disease. For example, a number of foreign publications have proven that the presence of the ACE2 gene polymorphism (rs2106809) indicates a predisposition to high blood pressure [8], as well as a lower risk of developing pulmonary hypertension [9]. It is worth noting that the dependence of hypertension development on the ACE2 gene polymorphism was not observed in patients of all ethnic groups. Thus, a study of women living in the south of Xinjiang showed a strong association of the SNP (rs2106809) with the development of hypertension [10], while the results of a study of Jordanians did not reveal such an association. [11].
ACE. The ACE gene is mapped to chromosome 17 (17q23), consists of 26 exons and encodes ACE, which has a significant effect on the regulation of blood pressure and electrolyte balance by converting angiotensin I to angiotensin II, which has a vasoconstrictor property and destroys bradykinin, the main vasodilator in the human body. Modern science knows more than 160 polymorphisms of this gene, the most significant of which is the insertion / deletion (I / D) of the ACE gene in various loci. SNP (rs1799752) has practical significance and the highest level of evidence. A number of authors have proven that patients with the DD genotype had a better reduction in blood pressure from enalapril and lisinopril than patients with the ID and II genotypes. The study also showed that the D allele is a genetic marker for the occurrence of hypertension [12]. Scientists have also proven that in patients with the DD genotype, in addition to the antihypertensive effect, the most pronounced regression of left ventricular hypertrophy is observed compared with I/D [13].
A large-scale study conducted among African Americans and including a comprehensive determination of ACE gene polymorphisms revealed that African Americans homozygous for rs4344 (12269GG or 12269AA) achieved target BP levels 2 times faster than heterozygous patients. In patients with SNP G20037A (rs4363), no association was observed between this polymorphism and the time to achieve target BP values. This study also revealed that the C17888T (rs4359) gene polymorphism leads to a faster response to ACE inhibitor therapy in homozygous patients (CC or TT) [14]. It has been shown that the AA/AT genotypes for rs4291 are associated with lower fasting glucose levels during lisinopril treatment in people with hypertension compared with the TT genotype for rs4291, which indicates the need to monitor the biochemical parameters of patients with this pathology (p = 0.001) [15].
In a study aimed at studying the metabolic status of patients taking ACE inhibitors, it was proven that homozygotes for the reference allele (AA) of the ACE gene (rs4329) showed lower Asp-Phe values (an indicator of the content of by-products of ACE activity on cholecystokinin) than homozygotes for the minor allele (GG). The level of excretion of Asp-Phe metabolites is associated with the effect on blood pressure, which allows us to consider them and other dipeptides as indicators of ACE activity [16]. Several modern studies have denied the relationship between the ACE gene polymorphism and the occurrence or severe course of hypertension. A study of the ACE gene polymorphism (rs4363) revealed that hypertension in some cases is associated with the socioeconomic status and lifestyle of patients. ACE polymorphisms could be associated with changes in the levels of triglycerides, urea, and glucose in patients with hypertension, but not be its direct predictor [17]. A study of the Thai patient population also did not reveal evidence of an association between gene polymorphisms (rs1799752, rs699, rs5186, rs1799998) associated with RAAS and hypertension [18]. The results of a systematic review including data from 2784 studies revealed a clear relationship between the ACE gene polymorphism (rs1799752) and the occurrence of hypertension in the African population. It has been shown that rs1799752 may be a potential genetic predictor of the development of hypertension in African Americans. In different African populations, a positive association of the genetic variants rs1799983, rs2070744, rs149868979, and rs61722009 of the NOS3 gene with the risk of hypertension development has been observed [19].
AGT. The AGT gene is located in the 1q42-q43 locus and contains 5 exons, encoding the angiotensinogen protein and the serum globulin alpha-globulin fraction. This gene is most important in the process of reducing blood pressure. Currently, more than 40 point mutations have been found in the gene. In patients with a polymorphism in the rs7079 gene of the AGT gene, taking benazepril, the most active reduction in DBP was observed in homozygotes of CC, compared with AS and AA [20].
A number of authors have shown that carriers of the GG genotype for rs5051 of the AGT gene respond more effectively to antihypertensive therapy using ACE inhibitors than carriers of the AA and AG genotypes, in connection with which it was suggested that genotyping for rs5051 could be used as a prognostic predictor of effective treatment. To date, no convincing data have been obtained on the predominant clinical significance of any allele [21]. The data on the study of the -217G>A polymorphism of the AGT gene are contradictory. According to the results of individual studies, it has been established that patients with the homozygous genotype -217AA of the AGT gene do not develop an antihypertensive effect due to the use of ACE inhibitors. Other studies have shown that people with at least 1 copy of the -217G and -20C alleles had a significant decrease in blood pressure, according to foreign data, as reported in the original genetic study [22].
Foreign studies have repeatedly established relationships between the clinical and morphofunctional features of hypertension in patients with AGT gene polymorphism, which contributed to the selection of the most effective therapy with ACE inhibitors [23].
Rysz J et al. provided data on the higher effectiveness of enalapril in patients who are carriers of the C allele of the -786T/C (rs2070744) polymorphic locus of the AGT gene and carriers of the T allele of the -665C/T (rs3918226) locus of the NOS3 gene [24].
AGTR1 и AGTR2.The AGTR1 gene is localized on the long arm of chromosome 3 (3q21-25) and encodes the angiotensin II receptor type 1. Changes in the structure of this gene lead to changes in the regulation of vascular tone and proliferation of the vascular wall, which is why its chromosomal changes cause cardiovascular pathologies. It has been shown that the GAG haplotype (includes SNPs G1675A (rs1403543) and G3726C of the AGTR2 gene and A1332G (rs5194 in AGTR1) showed a higher decrease in systolic blood pressure compared with the GAC haplotype and a group without GAG and GAC haplotypes [20, 25].
It has been proven that AGTR1 polymorphisms (rs275651, rs5182) are associated with a significant decrease in the incidence of myocardial infarction and cardiovascular mortality in patients with coronary heart disease taking perindopril [26].
It has now been established that the T allele of the AGTR1 gene (rs5182) is most associated with a decrease in the incidence of cardiovascular pathologies during treatment with ACE inhibitors in patients with coronary heart disease, compared with the C allele [27]. Similar results were obtained in a 12-year follow-up study conducted in China. Scientists have proven that the A allele of the AGTR1 gene (rs5186) is most associated with a decrease in the incidence of cardiovascular complications in the Asian population [28]. A large-scale EUROPA / PERGENE study revealed that the A and C alleles of the AGTR1 gene (rs275651, rs5182) are associated with a reduced response to perindopril in patients with coronary heart disease compared with the TT genotype [29].
SLCO1B1. It is also necessary to consider that genetic polymorphisms can affect not only the effectiveness of the therapy used but also the frequency of side effects and adverse drug reactions. One such gene is the SLCO1B1 gene, which encodes the OATP1B1 protein, which acts as a solute transporter and is localized on the basolateral membrane. During a large-scale study of the SLCO1B1 gene (rs4149056), several conclusions were made: the frequency of combined TC/CC genotypes was significantly higher in the group with complications and amounted to 31.7%. Second, in the presence of the TC genotype, the risk of developing an adverse drug reaction in the form of a dry cough increased by 2 times compared to the TT genotype, and the CC genotype gave an almost 6-fold risk of developing this adverse drug reaction; in carriers of the 521C genotype, the risk of cough was higher compared to the TT genotype, regardless of gender. In addition, a dependence of this side effect on the gene dose was established: the percentage of cough increased with an increase in the number of 521C alleles of the SLCO1B1 gene. For individuals who did not have the 521C allele, cough developed in 28.2%, for carriers of one 521C allele in 42.5%, and for carriers of two 521C alleles in 71.4% [30]. A study of the pharmacokinetics of temocapril in relation to genetic characteristics conducted in Japanese patients revealed that the concentration-time parameter (AUC) was lower in carriers of the *1b allele of the SLCO1B1 gene [31], which in the current nomenclature is designated as the SLCO1B1*37 allele (35230A>G (rs2306283, N130D) [32]. Recent data from pharmacogenetic studies indicate that in volunteers with SNP in SLCO1B1 and reduced function (DF), the area under the curve (AUC/DW), adjusted for dose/body weight, was approximately 1.7 times higher than that in volunteers with the normal function phenotype (NF) [33].
ABO. The ABO gene is located at 3q34.2 and encodes a glycosyltransferase that catalyzes the transfer of various carbohydrate groups to the H-antigen, forming antigens of the ABO system. Modern studies have shown that polymorphism in the ABO gene (rs495828) is clinically significant, considering the causes of cough from taking ACE inhibitors. Moreover, these polymorphisms turned out to be gender-specific since they were observed most often in women (p=0.0006; OR=3.26) [34]. A large-scale study of the dependence of ABO gene polymorphisms located at various loci and the risk of dry cough from taking enalapril revealed that SNPs in the gene are predictors of the occurrence of this side effect. The results showed that homozygous TT patients (rs495828) had a significantly increased risk of enalapril-induced cough, regardless of gender. The frequency of the polymorphic haplotype GATC (rs8176746, rs8176740, rs495828, rs12683493) was significantly higher in patients with cough (26.6%) than in the control group (18.8%) [35].
BDKRB1 и BDKRB2.The BDKRB1 and BDKRB2 genes are located on the long arm of chromosome 14 in close proximity at locus 14q32.2 and encode the bradykinin receptors B1 and B2. BDKRB1 is involved in vascular relaxation by stimulating the production of endothelial NO synthase and the subsequent generation of NO. The presence of the AA genotype for rs12050217 of the BDKRB1 gene is a predictor of a decrease in the fatality rate of cardiovascular pathologies compared with the AG and GG genotypes [27]. The BDKRB2 polymorphism can lead to changes in the blood supply to the skeletal muscles and a predisposition to diabetes mellitus, arterial hypertension, and osteoarthritis. A study of BDKRB1 (1098A/G) and BDKRB2 (-58T/C) polymorphisms in northern China found that the BDKRB2 (-58T/C) polymorphism was associated with an increased risk of hypertension when alleles were compared (p=0.01, OR=1.386, 95% CI [1.138–1.688]). A meta-analysis in the same population focusing on the BDKRB2 (-58T/C) polymorphism found a significant association between this polymorphism and hypertension in the entire study group [36]. The EUROPA/PERGENE study included 8726 patients, whose genotyping revealed that patients with the GG genotype for rs12050217 had a stronger pharmacological response to perindopril therapy than patients with the AA and AG genotypes [29].
ADRB2 и ADRB3. The β3-adrenergic receptor is an essential component of the sympathetic nervous system, the polymorphism of which can lead to impaired thermoregulation and lipolysis, as well as the development of diseases such as diabetes mellitus, hypertension, and obesity. A modern meta-analysis conducted among almost 10,000 people showed that SNP 190T>C (rs4994) of the ADRB3 gene, leading to the amino acid substitution Trp64Arg, is clinically significantly associated with the development of essential hypertension in Chinese and Caucasian populations [37]. Polymorphism in the β2-adrenergic receptor ADRB2 is associated with a change in the antihypertensive effect observed with the use of ACE inhibitors. A study by foreign scientists showed an association of the rs1042714GC genotype of the ADRB2 gene with the individual clinical response of patients from South Africa to enalapril [38]. It has been scientifically proven that the rs2053044(A/G) polymorphism of the ADRB2 gene can determine the blood pressure response to ramipril. The study obtained data indicating that patients with the GG genotype reached reference blood pressure values 12 days later than AA or AG carriers, which, adjusted for concomitant factors, took on average 2 times longer than the standard time [39].
NR3C2.The NR3C2 gene, localized on chromosome 4q31.1–31.2, encodes the mineralocorticoid receptor, which is present in cardiomyocytes. This gene has been studied less extensively because there is no data on its direct effect on the development of hypertension or the pharmacological response to ACE inhibitors. Using Sequenom MassArray technology, a study was conducted that revealed that the decrease in DBP from enalapril was significantly more pronounced in AA homozygotes compared to carriers of the AG+GG genotype for the rs5522 polymorphism (p = 0.009). In addition, for the rs2070950 polymorphism, the decrease in pressure in GG homozygotes compared to carriers of the GC+CC genotype was statistically insignificant (p = 0.065) [40].
MTHFR. Polymorphism of the MTHFR gene (C677T) of methylenetetrahydrofolate reductase is the main factor determining the state of hyperhomocysteinemia, which leads to endothelial dysfunction and affects the cardiovascular system. Single studies prove this hypothesis, but they do not allow us to form a clear idea of the effect of polymorphism on the outcome of therapy with ACE inhibitors. It has been proven that for patients at risk of developing coronary heart disease and hypertension, before prescribing ACE inhibitors and folic acid, it is necessary to screen genotypes in order to improve the clinical outcome of treatment [41].
PRCP. The human PRCP gene is located on chromosomal 11q14.1 and encodes the enzyme prolyl carboxypeptidase, which cleaves the proline-linked C-terminal amino acids in peptides such as angiotensin II, III, and des-Arg9-bradykinin. The importance of angiotensin II, one of the substrates of this enzyme, in the regulation of blood pressure and electrolyte balance suggests that this gene may be associated with essential hypertension. Inhibition of the PRCP gene may lead to the development of hypertension due to increased levels of angiotensin II and angiotensin III [42]. A poorly studied polymorphism of the prolyl carboxypeptidase gene (E112D) plays a role in the effectiveness of ACE inhibitors in maintaining reference blood pressure values. A study conducted among hypertensive patients from Huoqiu and Yuexi counties of Anhui Province, China, showed that patients with the EE genotype had greater reductions in both systolic and DBP than patients with ED or DD [43].
CYP11B2. The human CYP11B2 gene has a chromosomal localization of .8q24.3 and encodes aldosterone synthetase - cytochrome P450, catalyzing the 11-beta-hydroxylation of 11-deoxycorticosterone and its subsequent 18-hydroxylation to form aldosterone [44]. The CYP11B2 gene is also poorly studied in terms of its effect on the effectiveness of ACE inhibitors and the development of hypertension. Several foreign studies have confirmed that the -344C/T polymorphism affects the effectiveness of ACE inhibitors, whereas the A6547G polymorphism is not clinically significant. It was found that after 6 weeks of treatment, the decrease in DBP was significantly more pronounced in patients with the TT or CT genotype compared with patients with the CC genotype [45]. A number of drugs in modern medicine are prodrugs and acquire an active form only in the human body during the process of biotransformation. If in the case of conventional drugs, gene polymorphism can lead to a decrease in the effectiveness of therapy, then prodrugs may not be activated due to the polymorphism of the genes of the enzymes responsible for activation. Such enzymes include carboxyesterase 1, which is involved in the modification of enalapril into active enalaprilat.
CES1.The study of this enzyme is most widespread in modern medicine because it activates several prodrugs, including ACE inhibitors and the frequently prescribed enalapril. It was found that the polymorphic variant of CES1 (G143E) reduces the activation of ACE inhibitors, thereby affecting the therapeutic effect of the drug [46]. It has been reported that the polymorphisms G143E, L40T, G142E, G147C, Y170D and R171C can completely block the metabolism of enalapril, and Q169P, E220G and D269fs reduce the catalytic activity of the enzyme [47]. A study conducted at rest in healthy individuals showed that the average maximum decrease in systolic BP in the group who were not carriers of the G143E polymorphism was approximately 12.4% at the end of the study, compared with the baseline level (p = 0.001). In G143E carriers, no statistically significant reduction in blood pressure was observed [48].
Conclusion
Summarizing the analysis of literary sources on the effect of gene polymorphism on the efficacy, safety and risk of adverse reactions when using ACE inhibitors, it can be concluded that further studies are needed to compile guidelines for pharmacogenetic testing of patients before prescribing ACE inhibitors. Based on the results of the studies, it becomes obvious that many polymorphic alleles of genes can act as predictors of cardiovascular diseases, and, in particular, arterial hypertension. The use of genotyping in individual cases can help improve the effectiveness of treatment, especially in the presence of comorbid pathology and clinically significant drug interactions. However, with regard to routine practice, convincing data on the rationality of using this technology has not yet been obtained. The data obtained from domestic and foreign sources are quite variable and require further study and systematization.
References
1. Kolbin AS, Radaeva KS, Motrinchuk AS, Svechkareva IR. Trends in clinical pharmacology as represented by international and specialized journals. Kachestvennaya Klinicheskaya Praktika = Good Clinical Practice. 2024;(2):33-42. (In Russ.). https://doi.org/10.37489/2588-0519-2024-2-33-42. EDN: LYNKOF
2. Applied pharmacogenetics / ShP Abdullaev, AS Ametov, AV Boyarko [et al]. Moscow: Triada Publishing House, LLC, 2021. (In Russ.). ISBN 978-5-94789-982-5. EDN: PXWCTD.
3. Vasilevski IV. Drug – gene interaction and pharmacotherapeutic response. Mezhdunarodnye obzory klinicheskaya praktika i zdorovie. 2020;(1):7-19. (In Russ.). EDN: DCWUTP.
4. Moick S, Sommer I, Gartlehner G. WHO-Leitlinie: Leitfaden für die medikamentöse Behandlung von Bluthochdruck bei Erwachsenen [WHO Guideline for the Pharmacological Treatment of Hypertension in Adults]. Gesundheitswesen. 2023 Feb;85(2):139-142. German. https://doi.org/10.1055/a-1989-1745.
5. Drapkina OM, Vasilyeva LE. Debatable points of using angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists in patients with COVID-19. Cardiovascular Therapy and Prevention. 2020;19(3):2580. (In Russ.). https://doi.org/10.15829/1728-8800-2020-2580.
6. Chen YY, Liu D, Zhang P, et al. Impact of ACE2 gene polymorphism on antihypertensive efficacy of ACE inhibitors. J Hum Hypertens. 2016 Dec;30(12):766-771. https://doi.org/10.1038/jhh.2016.24.
7. Fan X, Wang Y, Sun K, et al. Polymorphisms of ACE2 gene are associated with essential hypertension and antihypertensive effects of Captopril in women. Clin Pharmacol Ther. 2007 Aug;82(2):187-96. https://doi.org/10.1038/sj.clpt.6100214
8. Fan Z, Wu G, Yue M, et al. Hypertension and hypertensive left ventricular hypertrophy are associated with ACE2 genetic polymorphism. Life Sci. 2019 May 15;225:39-45. https://doi.org/10.1016/j.lfs.2019.03.059.
9. Zi-Yang Y, Nanshan X, Dongling L, et al. ACE2 gene polymorphisms are associated with elevated pulmonary artery pressure in congenital heart diseases. Gene. 2023 Oct 5;882:147642. https://doi.org/10.1016/j.gene.2023.147642.
10. Luo Y, Liu C, Guan T, et al. Association of ACE2 genetic polymorphisms with hypertension-related target organ damages in south Xinjiang. Hypertens Res. 2019 May;42(5):681-689. https://doi.org/10.1038/s41440-018-0166-6. Epub 2018 Dec 12. Erratum in: Hypertens Res. 2019 May;42(5):744. https://doi.org/10.1038/s41440-019-0205-y.
11. Al-Eitan L, Al-Khaldi S, Ibdah RK. ACE gene polymorphism and susceptibility to hypertension in a Jordanian adult population. PLoS One. 2024 Jun 25;19(6):e0304271. https://doi.org/10.1371/journal.pone.0304271.
12. Heidari F, Vasudevan R, Mohd Ali SZ, et al. Association of insertion/ deletion polymorphism of angiotensin-converting enzyme gene among Malay male hypertensive subjects in response to ACE inhibitors. J Renin Angiotensin Aldosterone Syst. 2015 Dec;16(4):872-9. https://doi.org/10.1177/1470320314538878.
13. He H, Li LM, Cao WH, et al. A study of the relationships between angiotensin- converting enzyme gene, chymase gene polymorphisms, pharmacological treatment with ACE inhibitor and regression of left ventricular hypertrophy in essential hypertension patients treated with benazepril. Ann Hum Biol. 2005 Jan-Feb;32(1):30-43. https://doi.org/10.1080/03014460400027458.
14. Bhatnagar V, O'Connor DT, Schork NJ, et al. Angiotensin-converting enzyme gene polymorphism predicts the time-course of blood pressure response to angiotensin converting enzyme inhibition in the AASK trial. J Hypertens. 2007 Oct;25(10):2082-92. https://doi.org/10.1097/HJH.0b013e3282b9720e.
15. Irvin MR, Lynch AI, Kabagambe EK, et al. Pharmacogenetic association of hypertension candidate genes with fasting glucose in the GenHAT Study. J Hypertens. 2010 Oct;28(10):2076-83. https://doi.org/10.1097/HJH.0b013e32833c7a4d.
16. Altmaier E, Menni C, Heier M, et al. The Pharmacogenetic Footprint of ACE Inhibition: A Population-Based Metabolomics Study. PLoS One. 2016 Apr 27;11(4):e0153163. https://doi.org/10.1371/journal.pone.0153163.
17. da Agostini L, Cunha WR, Silva NNT, et al. Angiotensin-converting enzyme gene (ACE) polymorphisms are associated with dysregulation of biochemical parameters in hypertensive patients. Mol Biol Rep. 2023 Feb;50(2):1487-1497. https://doi.org/10.1007/s11033-022-08128-z.
18. Charoen P, Eu-Ahsunthornwattana J, Thongmung N, et al. Contribution of Four Polymorphisms in Renin-Angiotensin-AldosteroneRelated Genes to Hypertension in a Thai Population. Int J Hypertens. 2019 Aug 14;2019:4861081. https://doi.org/10.1155/2019/4861081.
19. Mabhida SE, Mashatola L, Kaur M, et al. Hypertension in African Populations: Review and Computational Insights. Genes (Basel). 2021 Apr 6;12(4):532. https://doi.org/10.3390/genes12040532.
20. Su X, Lee L, Li X, et al. Association between angiotensinogen, angiotensin II receptor genes, and blood pressure response to an angiotensinconverting enzyme inhibitor. Circulation. 2007 Feb 13;115(6):725-32. https://doi.org/10.1161/CIRCULATIONAHA.106.642058.
21. Yu H, Lin S, Zhong J, et al. A core promoter variant of angiotensinogen gene and interindividual variation in response to angiotensin-converting enzyme inhibitors. J Renin Angiotensin Aldosterone Syst. 2014 Dec;15(4):540-6. https://doi.org/10.1177/1470320313506481.
22. Woodiwiss AJ, Nkeh B, Samani NJ, et al. Functional variants of the angiotensinogen gene determine antihypertensive responses to angiotensinconverting enzyme inhibitors in subjects of African origin. J Hypertens. 2006 Jun;24(6):1057-64. https://doi.org/10.1097/01.hjh.0000226195.59428.57.
23. Shlyk SV, Drobotya NV, Khaisheva LA, et al. Effectiveness of personalized selection of antihypertensive therapy based on genetic polymorphism. European Heart Journal, Volume 41, Issue Supplement_2, November 2020, ehaa946.2760. https://doi.org/10.1093/ehjci/ehaa946.2760.
24. Rysz J, Franczyk B, Rysz-Górzyńska M, Gluba-Brzózka A. Pharmacogenomics of Hypertension Treatment. Int J Mol Sci. 2020 Jul 1;21(13):4709. https://doi.org/10.3390/ijms21134709
25. Fontana V, Luizon MR, Sandrim VC. An update on the pharmacogenetics of treating hypertension. J Hum Hypertens. 2015 May;29(5):283-91. doi: 10.1038/jhh.2014.76.
26. Brugts JJ, de Maat MP, Danser AH, Boersma E, Simoons ML. Individualised therapy of angiotensin converting enzyme (ACE) inhibitors in stable coronary artery disease: overview of the primary results of the PERindopril GENEtic association (PERGENE) study. Neth Heart J. 2012 Jan;20(1):24-32. doi: 10.1007/s12471-011-0173-6.
27. Brugts JJ, Boersma E, Simoons ML. Tailored therapy of ACE inhibitors in stable coronary artery disease: pharmacogenetic profiling of treatment benefit. Pharmacogenomics. 2010 Aug;11(8):1115-26. doi: 10.2217/pgs.10.103.
28. Lee JK, Wu CK, Tsai CT, et al. Genetic variation-optimized treatment benefit of angiotensin-converting enzyme inhibitors in patients with stable coronary artery disease: a 12-year follow-up study. Pharmacogenet Genomics. 2013 Apr;23(4):181-9. doi: 10.1097/FPC.0b013e32835a0ffa.
29. Oemrawsingh RM, Akkerhuis KM, Van Vark LC, et al. Individualized Angiotensin-Converting Enzyme (ACE)-Inhibitor Therapy in Stable Coronary Artery Disease Based on Clinical and Pharmacogenetic Determinants: The PERindopril GENEtic (PERGENE) Risk Model. J Am Heart Assoc. 2016 Mar 28;5(3):e002688. doi: 10.1161/JAHA.115.002688.
30. Luo JQ, He FZ, Wang ZM, et al. SLCO1B1 Variants and Angiotensin Converting Enzyme Inhibitor (Enalapril)-Induced Cough: a Pharmacogenetic Study. Sci Rep. 2015 Nov 26;5:17253. doi: 10.1038/srep17253.
31. Maeda K, Ieiri I, Yasuda K, et al. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan,and temocapril. Clin Pharmacol Ther. 2006 May;79(5):427-39. doi: 10.1016/j.clpt.2006.01.011.
32. Ramsey LB, Gong L, Lee SB, et al. PharmVar GeneFocus: SLCO1B1. Clin Pharmacol Ther. 2023 Apr;113(4):782-793. doi: 10.1002/cpt.2705.
33. Abbes H, Zubiaur P, Soria-Chacartegui P, et al. SLCO1B1 and ABCG2 genotype-informed phenotypes are related to variation in ramipril exposure. Basic Clin Pharmacol Toxicol. 2024 Sep;135(3):295-307. doi: 10.1111/bcpt.14046.
34. Mas S, Gassò P, Alvarez S, et al. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: the role of ACE, ABO, and BDKRB2 genes. Pharmacogenet Genomics. 2011 Sep;21(9):531-8. doi: 10.1097/FPC.0b013e328348c6db.
35. Luo JQ, He FZ, Luo ZY, et al. Rs495828 polymorphism of the ABO gene is a predictor of enalapril-induced cough in Chinese patients with essential hypertension. Pharmacogenet Genomics. 2014 Jun;24(6):306-13. doi: 10.1097/FPC.0000000000000050.
36. Gu W, Li Z, Wang Z, et al. Association of the bradykinin receptors genes variants with hypertension: a case-control study and meta-analysis. Clin Exp Hypertens. 2016;38(1):100-6. doi: 10.3109/10641963.2015.1060989.
37. Li YY, Lu XZ, Wang H, et al. ADRB3 Gene Trp64Arg Polymorphism and Essential Hypertension: A Meta-Analysis Including 9,555 Subjects. Front Genet. 2018 Apr 4;9:106. doi: 10.3389/fgene.2018.00106.
38. Masilela C, Pearce B, Ongole JJ, et al. Cross-sectional study of the association of 5 single nucleotide polymorphisms with enalapril treatment response among South African adults with hypertension. Medicine (Baltimore). 2021 Nov 19;100(46):e27836. doi: 10.1097/MD.0000000000027836.
39. Anthony EG, Richard E, Lipkowitz MS, Bhatnagar V. Association of the ADRB2 (rs2053044) polymorphism and angiotensin-converting enzyme-inhibitor blood pressure response in the African American Study of Kidney Disease and Hypertension. Pharmacogenet Genomics. 2015 Sep;25(9):444-9. doi: 10.1097/FPC.0000000000000154.
40. Luo JQ, Wang LY, He FZ, et al. Effect of NR3C2 genetic polymorphisms on the blood pressure response to enalapril treatment. Pharmacogenomics. 2014 Feb;15(2):201-8. doi: 10.2217/pgs.13.173.
41. Ma L, Zeng L, Wang X. MTHFR C677T gene polymorphism in patients with coronary heart disease and hypertension treated with enalapril and folic acid: implications for prognosis. Cell Mol Biol (Noisy-le-grand). 2024 Oct 8;70(9):142-147. doi: 10.14715/cmb/2024.70.9.20.
42. Wu Y, Yang H, Xiao C. Genetic association study of prolylcarboxypeptidase polymorphisms with susceptibility to essential hypertension in the Yi minority of China: A case-control study based on an isolated population. J Renin Angiotensin Aldosterone Syst. 2020 AprJun;21(2):1470320320919586. doi: 10.1177/1470320320919586.
43. Zhang Y, Hong XM, Xing HX, et al. E112D polymorphism in the prolylcarboxypeptidase gene is associated with blood pressure response to benazepril in Chinese hypertensive patients. Chin Med J (Engl). 2009 Oct 20;122(20):2461-5.
44. F. P. Guengerich Human Cytochrome P450 Enzymes. Chapter 10, P. 377-530. In:Cytochrome P450: Structure, Mechanism, and Biochemistry. Ed by P.R. Ortiz de Montellano, Kluwer Academic/Plenum Publishers, New York, 2005.
45. Yu HM, Lin SG, Liu GZ, et al. Associations between CYP11B2 gene polymorphisms and the response to angiotensin-converting enzyme inhibitors. Clin Pharmacol Ther. 2006 Jun;79(6):581-9. doi: 10.1016/j.clpt.2006.02.007.
46. Wang X, Wang G, Shi J, et al. CES1 genetic variation affects the activation of angiotensin-converting enzyme inhibitors. Pharmacogenomics J. 2016 Jun;16(3):220-30. doi: 10.1038/tpj.2015.42.
47. Hussain M, Basheer S, Khalil A, et al. Pharmacogenetic study of CES1 gene and enalapril efficacy. J Appl Genet. 2024 Sep;65(3):463-471. doi: 10.1007/s13353-024-00831-w.
48. Her LH, Wang X, Shi J, et al. Effect of CES1 genetic variation on enalapril steady-state pharmacokinetics and pharmacodynamics in healthy subjects. Br J Clin Pharmacol. 2021 Dec;87(12):4691-4700. doi: 10.1111/bcp.14888.
About the Authors
B. I. KantemirovaRussian Federation
Bela I. Kantemirova — PhD, Dr. Sci. (Med.), Professor, Professor of the Department of Pharmacology
Astrakhan
Competing Interests:
The authors declare that there is no conflict of interest.
O. V. Komarova
Russian Federation
Olga V. Komarova — Post graduate of the Department of Pharmacology
Astrakhan
Competing Interests:
The authors declare that there is no conflict of interest.
A. N. Romanova
Russian Federation
Aleksandra N. Romanova — Post-graduate of the Department of Pharmacology
Astrakhan
Competing Interests:
The authors declare that there is no conflict of interest.
What is already known about this topic?
- There is growing interest in personalized treatment approaches that consider the genetic characteristics of patients. Pharmacogenetic testing is used to select individual therapies, especially in patients with cardiovascular diseases.
- ACE inhibitors are widely used to lower blood pressure in patients with hypertension, but their efficacy and safety may vary depending on the patient’s genetic characteristics.
- The polymorphisms of genes associated with the renin-angiotensin-aldosterone system (RAAS) can affect the efficacy and safety of ACE inhibitors.
What is new in the article?
- The authors summarized data from various studies to identify clinically significant correlations between gene polymorphisms and the efficacy of ACE inhibitors.
- The article provides a detailed review of candidate genes (e.g., ACE, ACE2, AGT, AGTR1, SLCO1B1, etc.) that affect the pharmacological response to ACE inhibitors.
- New data are presented on how specific polymorphisms (e.g., rs2106809 in the ACE2 gene) can affect the effectiveness of therapy and the risk of side effects.
- It is emphasized that the impact of polymorphisms can vary depending on the ethnicity of the patients.
How can this affect clinical practice in the foreseeable future?
- The introduction of pharmacogenetic testing before prescribing ACE inhibitors can help doctors select a more effective and safe therapy for each patient.
- Taking into account the genetic characteristics of patients can reduce the incidence of unwanted side effects, such as dry cough, which often occurs when taking ACE inhibitors.
- Genotyping can help determine the optimal dosage of drugs, which is especially important for patients with comorbid conditions and chronic diseases.
- Based on the data obtained, new clinical guidelines can be developed that consider the genetic characteristics of patients for treating hypertension.
Review
For citations:
Kantemirova B.I., Komarova O.V., Romanova A.N. Pharmacogenetic features of angiotensin-converting enzyme inhibitors. Pharmacogenetics and Pharmacogenomics. 2024;(2):19-28. (In Russ.) https://doi.org/10.37489/2588-0527-2024-2-19-28. EDN: BXQLYJ










































